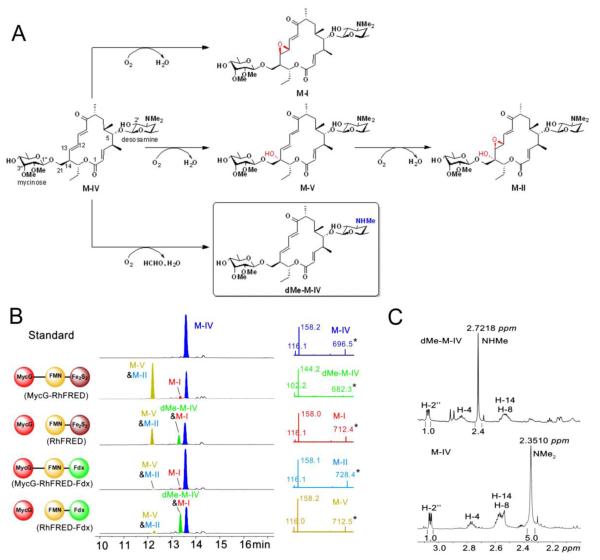
Figure 1.
Oxidation and demethylation of M-IV catalyzed by MycG using different redox systems (2 h reactions). (A) Scheme for MycG catalyzed reactions. The novel demethylation product dMe-M-IV is shown in box. The introduced hydroxyl and epoxy groups are labelled in red. The demethylated group is highlighted in blue. (B) Left panel: LC traces at 280 nm of different reaction extracts. The redox systems used are shown at the left side of the corresponding traces. Mycinamicin derivatives are colored differently for clarity. Due to close polarity, M-V and M-II were co-eluted; dMe-M-IV and M-I were co-eluted. Since M-I and M-II lacking of the diene moiety only have weak absorbance at 280 nm, the formation of products appear not to be proportional to substrate consumption. Right panel: MS/MS analysis of each mycinamicin compound (see Figure S1 for explanations of the secondary mass spectra). (C) Comparison of the 1H NMR spectra (see Figure S2 and S3 for full spectra) of M-IV and dMe-M-IV. The chemical shift and integrated peak area of the N-monomethyl group in dMe-M-IV are apparently different from those of the N-dimethyl group in M-IV.