Abstract
(−)-Epigallocatechin-3-gallate (EGCG) is one of the major polyphenol components in green tea. It effectively induces apoptosis in prostate cancer cells. The anticancer effect of this reagent is appealing because it is a natural component of a popular daily beverage that has proven harmless for thousands of years, making it a good candidate chemopreventive agent. EGCG suppresses cell growth and causes cell death, but the mechanisms are not well characterized, especially in androgen-independent prostate cancer cells. In the present study, using Affymetrix genechip Hu133 2.0, we analyzed the gene expression patterns of the androgen-independent prostate cancer cell line Du145, treated with or without EGCG, and found 40 genes whose expression levels were altered (>twofold, either upregulated or downregulated, P < 0.01) upon treatment with EGCG. These gene products are involved in the functions of transcription, RNA processing, protein folding, phosphorylation, protein degradation, cell motility, and ion transport. Among them, inhibitor of DNA binding 2 (ID2), known as a dominant anti-retinoblastoma (Rb) helix-loop-helix protein, was found to be downregulated fourfold by EGCG treatment. Forced expression of ID2 in Du145 cells reduced apoptosis and increased cell survival in the presence of EGCG, and knockdown ID2 expression in Du145 cells using a morpholino oligonucleotide specific for ID2 mimicked the apoptosis effect generated by EGCG treatment, although it was milder. To our knowledge, this is the first report indicating that ID2 is one of the critical factors in the signaling pathway of Du145 cell death induced by EGCG.
Prostate cancer is one of the leading causes of cancer death for men in developed countries; almost one in every six men develop this disease. It mostly occurs at an advanced age of over 65 years and is more prevalent as men get older. A large number of prostate cancer cases are diagnosed each year through annual serum prostate-specific antigen (PSA) screening. Although the disease shows very slow progression,(1) almost 30 000 men die of the disease, annually. In the early stage, growth of prostate cancer cells depends on androgen. Castration or other androgen-ablation treatment has been effective in controlling the progression of tumors. However, androgen receptor mutation and amplification may cause prostate cancer resistance to hormonal ablation.
The high prevalence of prostate cancer in Western countries has been attributed to a variety of factors, including age, race, genetics, and environmental factors or diets. The difference in dietary habits has been suggested to explain the relatively low incidence of prostate cancer in Far East Asia, such as in Japan and China, versus those in America, as tea is the most popular beverage among Chinese and Japanese people. It turns tea into the focus of epidemiological studies for cancer chemoprevention. (−)-Epigallocatechin-3-gallate (EGCG), the main polyphenol component of tea, has been considered a cancer-preventive agent for its apoptotic and growth-inhibitory effects on cancer cells in vitro and in animal models over the past several years.(2) EGCG plays a critical role in cell growth arrest and enhancement of apoptosis in tumors of a variety of organs and tissues, such as nasopharyngeal carcinoma,(3) cervical cancer cell lines,(4) bladder tumor cells,(5) prostate cancer cell lines,(6) liver cancer cell line,(7) gastrointestinal tract,(8) and lung and mammary cancer cell lines.(9) Some of the anticancer effects of EGCG may result from its protection of DNA from methylation and activation of silenced genes.(10) EGCG also increases the expression of p53 (wild type), p21/wild type P53-activated fragment 1 (WAF1) protein, and Bcl2-associated X protein (Bax)/B-cell lymphoma 2 (Bcl-2) protein in LNCaP prostate cancer cells, leading to cell cycle arrest and apoptotic cell death.(11,12) EGCG activates ERK1/ 2 via the phosphoinositide-3-kinase (PI3-K)-dependent signaling pathway to inhibit the cell proliferation of PC3 prostate cancer cells, but not in RWPE-1, an immortalized prostate cell line.(13) Du145 is an androgen-independent prostate cancer cell line that is insensitive to hormone ablation and has distinctive metastatic behavior from that of PC3 and LNCaP cells. EGCG was shown to suppresses Du145 cell growth and induce apoptosis through an increase in reactive oxygen species and induction of mitochondrial depolarization, without changing the expression of BCL-2, BCL-XL, and Bcl 2 antagonist of cell death (BAD).(14)
Despite our knowledge about the anticancer effects of EGCG, the molecular mechanism of EGCG on the androgen receptornull prostate cancer cell line Du145 is still fragmented and not well characterized. In the present study, we investigated the EGCG-regulated gene expression effect on Du145 using Affymetrix (Santa Clara, CA, USA) gene chip U133 2.0. By comparing the gene expression levels of Du145 cells treated with and without EGCG, we found that inhibitor of DNA binding 2 (ID2) was dramatically downregulated by EGCG. Restoration of ID2 expression showed protection against cell death induced by EGCG. Downregulation of ID2 without treatment of EGCG mimicked the EGCG effect on Du145 cells.
Materials and Methods
Cell culture and treatment
The prostate cancer cell lines Du145 and LNCaP were purchased from American Type Culture Collection (Manassas, VA, USA). Du145 was cultured with modified Eagle’s medium, and LNCaP with RPM 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Mediatech, Manassa, VA, USA) at 37°C and 5% CO2. When cultured cells reached 80–90% confluence, EGCG (Cayman Chemical, Ann Arbor, MI, USA) (30 mg/mL in PBS or water) was added to the culture medium to reach the final concentrations of 20–120 µm. Tea extract was prepared by heating Japanese green tea leaves (Ujino Tsu Yuseicha Co., Kyoto, Japan) in water, boiling for 2 min, keeping them warm in the water for 15 min, then adjusting the pH to 7.30 and further filtering them through a Millex-OR filter (0.2 µm) (Millipore, Bedford, MA, USA). The tea extract was then reconstituted in PBS buffer. Up to 3% tea extract was added to the culture medium for treatment of cells.
Affymetrix chip analysis
cRNA preparation
Total RNA was extracted from Du145 cells treated with or without EGCG, and purified with a Qiagen RNeasy kit (Qiagen, San Diego, CA, USA). Five micrograms of total RNA was used in the firststrand cDNA synthesis with Superscript II (Invitrogen) primed with T7 promoter-tagged (dT)-24 primer (GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGG-[dT]24). The second-strand cDNA synthesis was carried out at 16°C for 2 h in a reaction containing RNAse H and Escherichia coli DNA polymerase I DNA ligase. T4 DNA polymerase was added to the reaction for an additional 5-min incubation to blunt the ends of synthesized cDNA. The purified double-stranded cDNA was used as a template in in vitro transcription to produce biotinlabeled cRNA using the MEGAscript system (Ambion, Austin, TX, USA).
Affymetrix chip hybridization
Fifteen micrograms of cRNA was fragmented by incubation in a buffer containing 200 mm Tris-acetate pH 8.1, 500 mm KOAc, and 150 mm MgOAc at 95°C for 35 min. The fragmented cRNA was then hybridized with a pre-equilibrated Affymetrix Hu133 2.0 chip at 45°C for 14–16 h. After the hybridization cocktails were removed, the chips were then washed in a fluidic station with low-stringency buffer (6× sodium chloride and sodium phosphate mono basic [SSPE], 0.01% Tween 20, 0.005% antifoam) for 10 cycles (two mixes/cycle), and stringent buffer (100 mm 4-Morpholineethanesul fonic Acid [MES], 0.1 M NaCl, 0.01% Tween 20) for four cycles (15 mixes/cycle), and stained with strepto–avidin phycoerythrin (SAPE; Molecular Probes, Eugene, OR, USA). This was followed by incubation with biotinylated mouse anti-avidin antibody, and restaining with SAPE. The chips were scanned in an Affymetrix genechip scanner 3000 to detect the hybridization signals.
pCMV-ID2 construct and cloning
The full-length sequence of ID2 was excised with KpnI and XhoI from a pBluescriptR clone (Id 4820416; Open Biosystem, Bethesda, MD, USA) and ligated to a similarly restricted pCMVscript vector using T4 DNA ligase. The pCMV-ID2 was then transformed into TOP 10 competent cells (Invitrogen), and colonies containing pCMV-ID2 were selected. The pCMV-ID2 was sequenced to rule out mutation. Clones of pCMV-ID2 or pCMVscript free of mutation were then transiently transfected into Du145 cells with Superfect reagent (Qiagen, Valencia, CA, USA). The cells transfected with pCMVscript were used as controls. Twenty-four hours after the transfection, EGCG or green tea was added to the culture medium. Cells were harvested 3 days after the treatment and subjected to further analysis.
Semiquantitative RT-PCR
Total RNA was prepared from the cells transfected with pCMV-ID2 or pCMV script, and was used as a template to generate the first-strand cDNA through random priming and reverse transcription with Superscript II. The first-strand cDNA was then diluted 1:10, 1:100, 1:1000, 1:10 000 (20 ng/µL, 2 ng/µL, 200 pg/µL, and 20 pg/µL). PCR was carried out using primers specific for ID2 (gacacaagcctactgaatgctg/gcagaaatagacatctctgccac) or β-actin (tcaagatcattgctc ctcctgagc/tgctgtcaccttcaccgttccagt) for 30 cycles of 94°C for 30 s, 60°C for 3 min, followed by 72°C for 10 min.
FACS analysis of apoptotic cells
The cells at 80% confluence were treated without or with 20–80 µm EGCG for 3 days and then subjected to apoptosis analysis. For the cells with forced expression of ID2, cells were treated with 80 µm EGCG 2 days after transfection with pCMV-ID2 or pCMVscript. These cells were trypsinized 3 days later, and washed three times with PBS. The single-cell suspension of the harvested cells in 100 µL binding buffer was incubated with 5 µL Alexa Fluor 488-conjugated Annexin V and propidium iodide 1 µL (100 µg/mL) for 20 min in the dark at room temperature (Invitrogen). FACS analysis was subsequently carried out on a LSR II flow cytometer (BD Bioscience, San Jose, CA, USA). The cells without staining were used as negative controls, and the cells irradiated with 300 millijoule (mj) of UV radiation was used as a positive control. The UV-treated cells stained with either Alexa Fluor 488 or propidium iodide were used to calibrate the system. All of the samples were prepared in triplicate.
Morpholino siRNA inhibition of ID2 expression
A morpholino oligonucleotide specific for ID2 (CTTTCATGCTGACCGCGAGGGAA) or scramble control (5′-TAATGTATTGGAACGCATATT) was added to an electroporation cuvette (2 mm). The Du145 cell pellet was resuspended in 200 µL of Opti-MEM buffer (Invitrogen, Carlsbad, CA, USA) and then transferred to an electroporation cuvette containing morpholino ID2 or scramble control. Electroporation was carried out in the GenePulser Xcell (Bio Rad Laboratories, Hercules, CA, USA) with one square pulse of 150 V for 25 ms. After the electric shock, the cells were transferred to a culture dish with warm MEM medium containing 10% FBS. Forty-eight hours later, cells were harvested to examine ID2 expression, and to examine cell death through FACS analysis of AnnexinV binding (72 h later).
Immunoblot detection of ID2, Rb, phospho-Rb
To examine ID2 expression, Du145 cells were treated with 80 µm EGCG or 3% tea for 2 days, and then harvested by scraping. The cells were pelleted, then lysed with RIPA buffer (50 mm Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 µg /mL each of aprotinin, leupeptin, pepstatin, and 1 mm Na3VO4). The lysates were centrifuged to remove the insoluble cell debris. The proteins were resolved by 7% SDS-PAGE and blotted onto PVDF membrane. The membrane was blocked with 5% skim milk in Tris–Tween 20 buffer, pH 7.6 (TBS-T) for 1 h at room temperature, followed by 1 h of incubation with primary rabbit anti-ID2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-Rb or rabbit antiphospho- Rb antibodies. The membrane was then washed three times with TBS-T buffer and incubated with horseradish peroxidase-conjugated secondary antibody specific for rabbit (ID2), mouse (Rb), and rabbit (phospho-Rb) for 1 h at room temperature. The proteins of interests were detected with the ECL system (GE Life Sciences, Piscataway, NJ, USA) according to the manufacturer’s protocols.
Results
Du145 cell death induced by EGCG and green tea
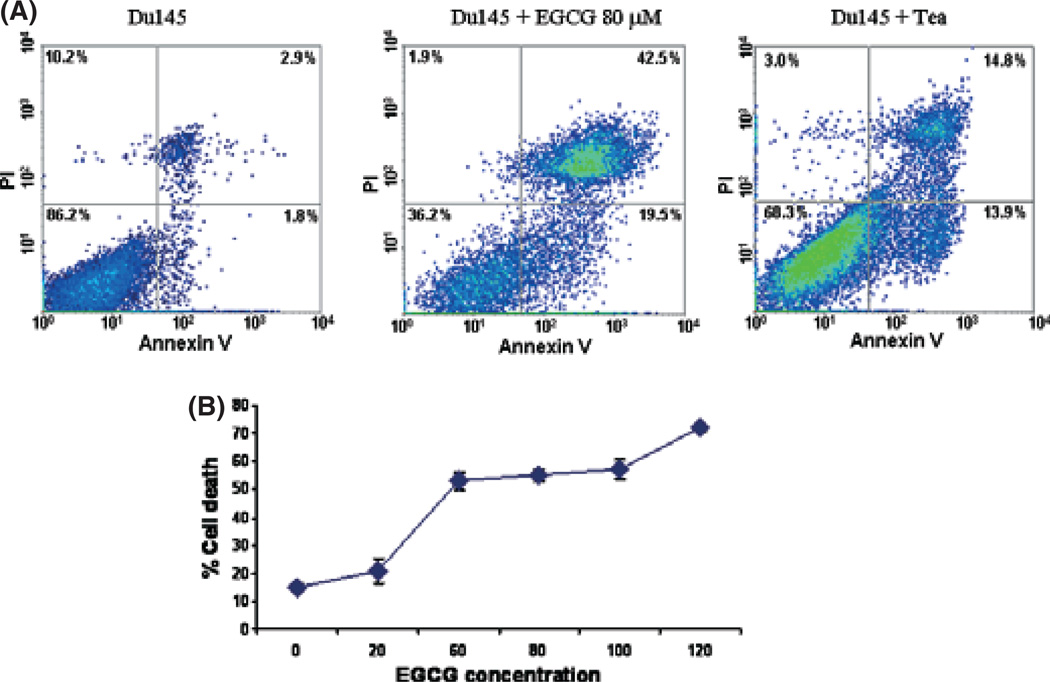
EGCG is the most abundant polyphenol in tea, and is thought to be the main component in tea exhibiting anti-cancer effects. To investigate the mechanism of EGCG-induced cell death in prostate cancer cells, the prostate cancer cell lines Du145 and LNCaP were incubated with EGCG or green tea. Three days after treatment, cell death was then examined by trypan blue (data not shown) and quantified by FACS analysis of Annexin V and propidium iodide binding. As translocation of phosphatidylserine from the inner leaflet of membrane to the outer membrane is an early event of apoptosis, detection of such translocation through binding of Annexin V to the intact cell membrane in FACS analysis indicates cells undergoing apoptosis. On the other hand, propidium iodide readily detects cell necrosis by penetrating through leaky cell membranes and binding to genomic DNA. When cells are stained with both Annexin V and propidium iodide, they are in the late stages of cell apoptosis. As shown in Figure 1 and Table 1, treatment with EGCG (20–120 µm) increased Du145 cell death by up to fourfold, in a dose-dependent manner. Most of these cell deaths were the result of apoptosis, either in early (19.5%) or late (42.5%) stages. Killing of LNCaP cells by EGCG, however, was less dramatic (Table 1). When cells were treated with tea extract, similar findings were identified (Fig. 1).
Fig. 1.
Du145 cell death induced by (−)epigallocatechin-3-gallate (EGCG) and green tea. (A) Representative samples of FACS analysis of cell death induced by EGCG or tea. DU145 cell samples treated without (left) or with 80 µm EGCG (middle) or with 3% green tea (right) were stained with propidium iodide (PI) and Alexa Fluor 488-labeled annexin V. (B) EGCG dose-dependent cell death of Du145. Du145 cells were treated with 0–120 µm EGCG for 3 days, and subjected to FACS analysis as in (A). Triplicate experiments were carried out for each concentration of EGCG. Standard deviations are indicated.
Table 1.
(−)-Epigallocatechin-3-gallate (EGCG)-mediated cell death
| Cells and treatment | PI staining alone (%) |
Annexin V alone (%) |
Annexin V + PI (%) |
|---|---|---|---|
| Du145 | 1.63 ± 0.12 | 6.93 ± 0.37 | 6.87 ± 0.65 |
| Du145 + EGCG (80 µm) | 1.87 ± 0.38 | 19.4 ± 1.26 | 41.13 ± 1.19 |
| Du145 + ea | 3.03 ± 0.45 | 14.6 ± 0.57 | 15.27 ± 1.91 |
| LNCaP | 11.3 ± 0.73 | 2.7 ± 1.3 | 7.2 ± 0.53 |
| LNCaP + EGCG (80 µm) | 21.77 ± 1.35 | 11.77 ± 1.78 | 10.93 ± 1.70 |
| Du145 + pCMVscript | 3.5 ± 0.24 | 8.07 ± 0.63 | 11.27 ± 0.69 |
| Du145 + pCMVscript + EGCG (80 µm) | 4.77 ± 0.29 | 14.33 ± 0.66 | 39.43 ± 0.82 |
| Du145 + pCMV-ID2 | 3.83 ± 0.29 | 6.93 ± 0.41 | 9.03 ± 0.21 |
| Du145 + pCMV-ID2 + EGCG (80 µm) | 9.63 ± 0.90 | 6.6 ± 0.41 | 12.13 ± 0.61 |
| Du145 + siScramble | 0.9 ± 0.08 | 7.07 ± 0.32 | 12.03 ± 0.45 |
| Du145 + siID2 | 1.23 ± 0.17 | 13.47 ± 0.25 | 11.77 ± 0.34 |
The numbers represent the average of three to six experiments. Standard deviations are indicated. PI, propidium iodide.
Gene expression alterations in Du145 induced by EGCG
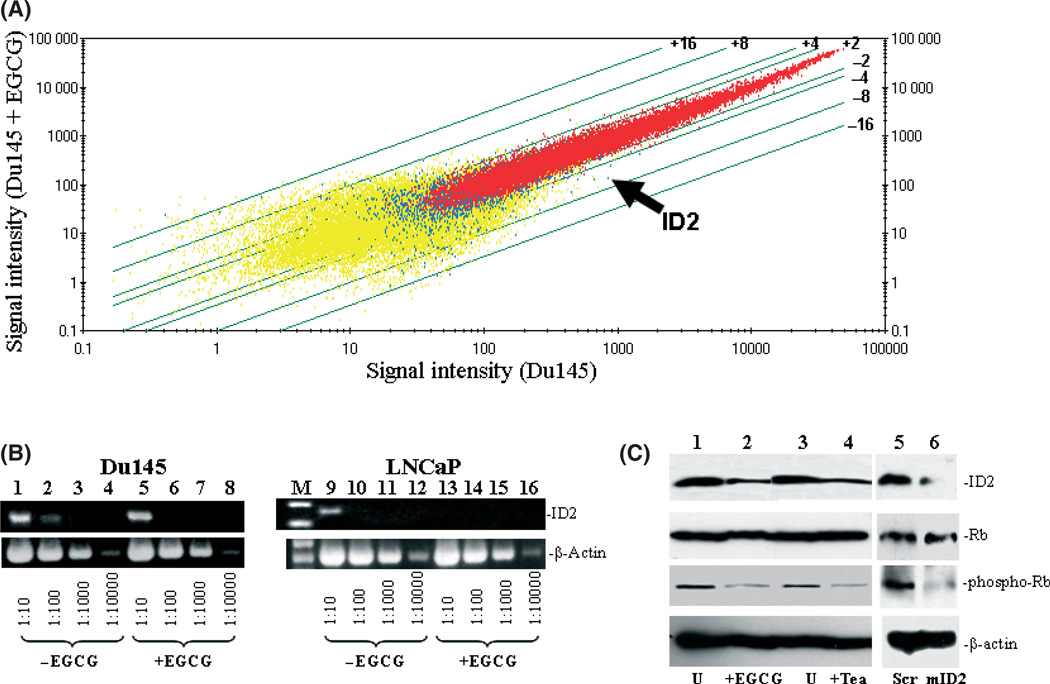
To identify the critical signaling molecules mediating EGCG-induced cell death in Du145 cells, we carried out gene expression analyses of Du145 cells treated with or without EGCG utilizing Affymetrix Hu133 2.0 chips. The signal intensity data were subsequently analyzed to identify genes whose expression was most significantly altered in DU145 cells after EGCG treatment. A list of the top 40 genes with the most altered expression by EGCG is shown in Table 2. The top 20 genes upregulated by EGCG includes genes that are involved in nutrient or ion transport, cell motility, phosphorylation, and inflammatory response, whereas among the top 20 downregulated genes include those that are mostly involved in transcription regulation, proteolysis, and cellular defense systems (Table 2; Fig. 2A). Among the downregulated genes, ID2, a dominant negative helix-loop-helix protein that inactivates Rb protein and thus allows the cell cycle to progress, was downregulated fourfold when Du145 was treated with EGCG (P = 2 × 10−5, baseline matrix analysis). To validate the microarray results, semiquantitative RT-PCR was carried out on cDNA templates obtained from Du145 and LNCaP cells treated with or without EGCG, using primers specific for ID2 and β-actin. As shown in Figure 2(B), EGCG treatment downregulated ID2 mRNA in both DU145 and LNCaP cells by approximately 10-fold, largely confirming the accuracy of Affymetrix array analysis.
Table 2.
Gene expression alterations in (−)-epigallocatechin-3-gallate-treated cells
| Gene symbol |
Fold change |
P-value | Function |
| MKLN1 | 10.56 | 0.000618 | Cell motility |
| NUP188 | 9.85 | 0.000307 | Transport |
| EST | 7.46 | 0.001077 | NK |
| ITGB6 | 6.50 | 0.001486 | Inflammatory response |
| TFRC | 4.92 | 0.00002 | Iron ion transport |
| SFRS11 | 4.00 | 0.001077 | mRNA processing |
| LOC284952 | 3.25 | 0.000114 | NK |
| KITLG | 3.25 | 0.000438 | Cell adhesion |
| NUCKS1 | 3.25 | 0.00002 | NK |
| TRUB1 | 3.25 | 0.000438 | tRNA processing |
| MBNL1 | 3.03 | 0.00002 | Embryonic development |
| AHCTF1 | 3.03 | 0.000088 | Transcription |
| C18orf25 | 3.03 | 0.000966 | NK |
| CLIC4 | 3.03 | 0.000046 | Transport |
| CLIC4 | 2.83 | 0.00002 | Transport |
| ZFX | 2.83 | 0.000068 | Transcription |
| DHX9 | 2.83 | 0.000088 | NK |
| BUB1 | 2.83 | 0.000273 | Protein amino acid phosphorylation |
| RP6-213H19.1 | 2.83 | 0.00002 | Protein amino acid phosphorylation |
| LASS6 | 2.83 | 0.000027 | Regulation of transcription |
| PPIL4 | −21.11 | 0.000167 | Protein folding |
| KLRC4 | −17.15 | 0.001486 | Cellular defense response |
| EST | −8.57 | 0.000692 | NK |
| EST | −6.50 | 0.001201 | NK |
| EST | −6.06 | 0.001651 | NK |
| ZNF224 | −6.06 | 0.001336 | Transcription |
| EST | −4.92 | 7.8E-05 | NK |
| ID2 | −4.00 | 2E-05 | Negative regulation of transcription |
| EST | −2.83 | 0.000966 | NK |
| BACE1 | −2.46 | 0.000101 | Proteolysis |
| XAB2 | −2.46 | 2E-05 | Blastocyst development |
| EST | −2.46 | 0.000692 | NK |
| EST | −2.46 | 0.001336 | NK |
| IREB2 | −2.30 | 0.001651 | Cellular iron ion homeostasis |
| ZNF250 | −2.30 | 0.000618 | Transcription |
| ZNF574 | −2.14 | 8.9E-05 | Transcription |
| HK2 | −2.14 | 0.000692 | Glycolysis |
| KIAA1542 | −2.14 | 0.000273 | NK |
| EST | −2.14 | 0.000241 | NK |
| CSPP1 | −2.14 | 0.000167 | NK |
NK, not known.
Fig. 2.
Downregulation of inhibitor of DNA binding 2 (ID2) in Du145 by (−)-epigallocatechin-3-gallate (EGCG). (A) Affymetrix Hu133 2.0 gene chip analysis was carried out to analyze the gene expression alteration of Du145 treated with 80 µm EGCG for 3 days. Scatter plots of the signal intensities of over 54 000 probe sets of Du145 cells treated with EGCG versus those from cells without treatment were shown. Lines represent cut-offs of the indicated fold changes (≥twofold). Arrow indicates the plot of the ID2 gene. (B) Semiquantitative RT-PCR of the ID2 gene in Du145 and LNCaP cells. Du145 or LNCaP cells were treated with or without EGCG. cDNA (200 ng) from Du145 (left) and LNCaP (right) was titrated to the indicated dilutions. PCR were carried out using primers specific for ID2 or β-actin. The PCR products were then electrophoresed and visualized in 2% agarose gel. (C) Immunoblotting analysis of ID2 and Rb1 phosphorylation in Du145 cells. Proteins from Du145 cells treated with 80 µm EGCG (or 3% tea), or 3% PBS (U), or scramble (Scr) or ID2-specific (mID2) morpholino oligonucleotides were extracted, electrophoresed in 7% SDS-PAGE gel, and immunoblotted with the indicated antibodies.
ID2 mediates Du145 cell death induced by EGCG
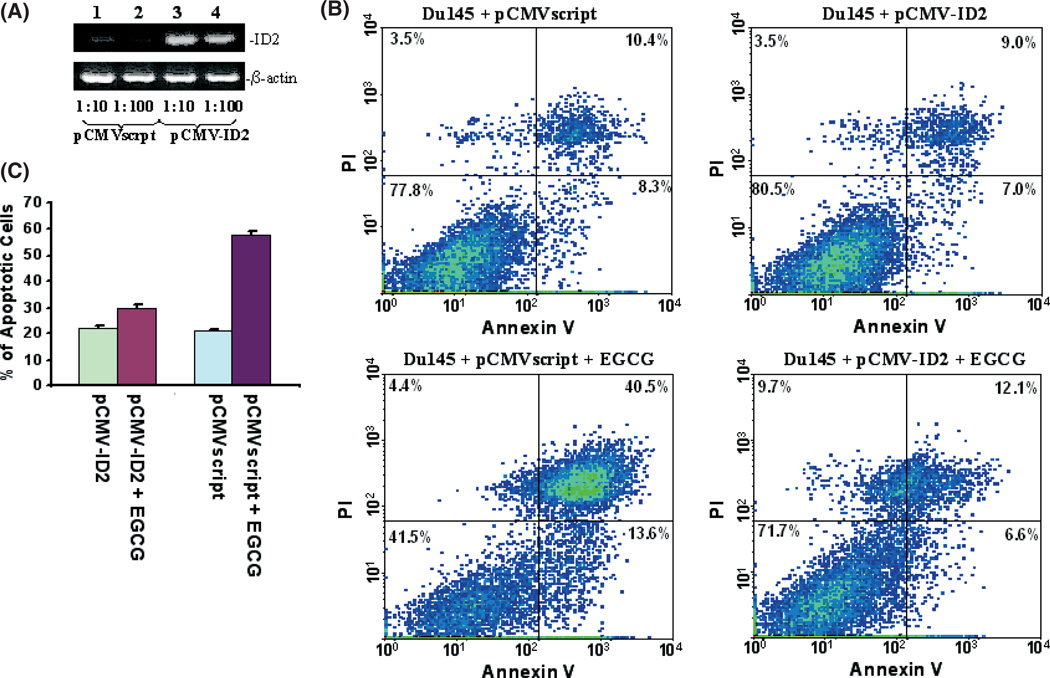
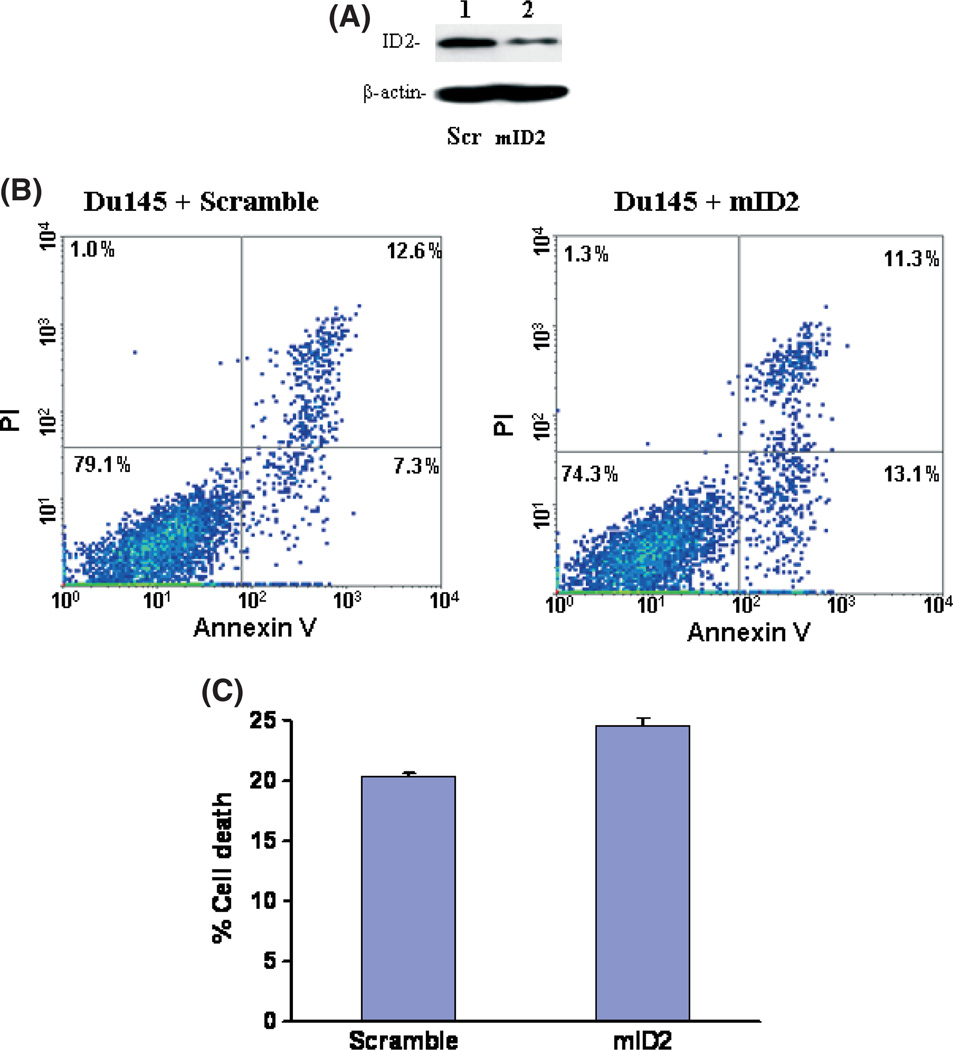
As ID2 contains inhibitory activity on the tumor-suppressor activity of Rb molecules, immunoblotting analyses were subsequently carried out to investigate whether downregulation of ID2 reduces phosphorylation of Rb. As shown in Figure 2(C), downregulation of ID2 protein had no effect on the expression of Rb. However, downregulation of ID2 induced a dramatic reduction in the phosphorylation level of Rb molecules, indicating an increased cell cycle checkpoint activity of Rb. Interestingly, treatment with 3% tea induced a similar reduction in the phosphorylation of Rb molecules, suggesting that both EGCG and tea contain the same signaling activity to suppress ID2 activity. Downregulation of ID2 expression using a morpholino oligonucleotide also produced a similar decrease in Rb phosphorylation without having an impact on Rb expression (Fig. 2C). In order to investigate the role of ID2 in EGCG-induced cell death, ID2 was inserted into the eukaryotic expression vector pCMVscript. This expression vector (pCMV-ID2) was transfected into Du145 cells to overexpress ID2. The transfected cells were then treated with or without EGCG for 4 days. As shown in Figure 3, Du145 cells transfected with pCMV-ID2 showed greater than twofoldreduction in cell death caused by EGCG in comparison with cells transfected with pCMVscript control vector, suggesting a protective role of ID2 against cell death caused by EGCG. Interestingly, expression of ID2 practically eliminated the apoptosis induced by EGCG (Fig. 3C), but had a limited role in reducing necrotic cell death. This suggests that multiple mechanisms are involved in EGCG-induced cell death, and downregulation of ID2 is the main factor in inducing apoptosis. To investigate whether downregulation of ID2 by itself is sufficient in inducing cell death, a morpholino oligonucleotide specific for the ID2 translation start site was transfected into Du145 cells to specifically knockdown the expression of ID2. As shown in Figure 4, treatment with the ID2 morpholin oligonucleotide produced a significant reduction in ID2 expression in Du145 cells, and a 1.9-fold increase in early apoptosis, but had no impact on necrotic cell death, supporting ID2 being a critical component in EGCG-induced and to some extent tea-induced cancer cell death.
Fig. 3.
Inhibitor of DNA binding 2 (ID2) overexpression rescues Du145 cells from apoptosis. (A) Semiquantitation of ID2 expression by RT-PCR on RNA from Du145 cells transfected with pCMVscript (lanes 1 and 2) or pCMV-ID2 (lanes 3 and 4). cDNA templates (200 ng) from Du145 transiently transfected with pCMV script or pCMV-ID2 were titrated to the indicated dilutions. PCR were carried out using primers specific for ID2 or β-actin. The PCR products were then electrophoresed and visualized in 2% agarose gel. (B) Representative samples of FACS analysis of Du145 transfected with pCMV-ID2 or pCMVscript, and treated with or without (−)-epigallocatechin-3-gallate (EGCG). (C) Means of DU145 cell deaths by FACS of (B). Each condition was triplicated in the analysis. Standard deviations are indicated.
Fig. 4.
Suppression of inhibitor of DNA binding 2 (ID2) in Du145 cells induces cell death. (A) Immunoblotting analysis of ID2 expression in Du145 cells. Protein extracts from Du145 cells transfected with the indicated morpholino oligonucleotides were electrophoresed in 7% SDS-PAGE gel and immunobloted with antibodies specific for ID2 and β-actin. (B) Representative samples of FACS analysis of Du145 cells transfected with morpholino oligonucleotides specific for ID2 (mID2) and scramble control (Scr). (C) Means of DU145 cell deaths by FACS quantification of Du145 cells transfected with Scr control or mID2. Each condition was carried out in triplicate. Standard deviations are indicated.
Discussion
EGCG is the most abundant and active catechin in tea and has been demonstrated to reduce the risk of cancers, such as lung cancer,(15) skin cancer,(16) and breast cancer,(17) caused by carcinogens in both humans and animals. EGCG inhibits the activity of DNA methyltransferase to prevent DNA methylation and protects genomic DNA from damage.(10) EGCG inhibits epidermal growth factor receptor (EGFR) phosphorylation, suppresses transforming growth factor (TGF)-α-induced serine/threonine protein kinase murine thymoma viral oncogene homolog (AKT) and signal transducer and activator of transcription (STAT3) activities, and modulates the nuclear factor kappa-B (NF-κB) and hepatocyte growth factor signaling pathways. One study suggested that EGCG induces cell death through stabilization of the p53 protein.(11) However, the present study indicates for the first time that downregulation of the cell cycle facilitator ID2 by EGCG plays a critical role in tea-mediated apoptosis. ID2 is a member of the ID family of proteins, involved in the promotion of cell proliferation, survival, and suppression of cell differentiation.(18) ID2 binds to pRB (retinoblastoma tumor suppressor protein), which is involved in cell cycle regulation and tumor suppression. Downregulation of ID2 allows retinoblastoma (Rb) to induce cell growth arrest and cell death. Two lines of evidence support that ID2 downregulation is essential for EGCG-mediated cell death: First, reversal of downregulation of ID2 by expressing ID2 through cytomegalovirus immediate early promoter abrogates the apoptotic effect of EGCG, even though overexpression of ID2 in Du145 without EGCG results in no significant change in cell survival. Second, downregulation of ID2 without EGCG treatment increases death of Du145 cells (Fig. 4C), albeit much milder. These findings were corroborated with reduced phosphorylation of Rb molecules when ID2 is downregulated.
The implications of this study are twofold. First, it indicates that the critical target of EGCG signaling in the prostate cancer cell line Du145 is activation of the Rb molecule, through concerted signaling of downregulation of its antagonist (ID2) and upregulation of its agonists (stabilization of p53).(7) Second, this study shows that targeting ID2 could be a possible approach for therapeutic interventions of prostate cancer treatment, that is, sensitizing cancer cells to apoptosis. Because ID2 expression is independent of p53, the signaling pathway leading to ID2 transcription activation remains unclear. Future studies identifying molecules that regulate ID2 transcription will further shed light on the mechanism of how EGCG, and to some extent tea, induces cancer cell death.
Acknowledgements
We thank Chia-Yue Yen for technical support. This study was in part supported by grants from the American Cancer Society (RSG-08-137-01-CNE) and National Cancer Institute (RO1 CA098249 and R56 CA098249).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Milacic V, Chen MS, et al. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol. 2008;23:487–496. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Yang LF, Ye M, Gu HH, Cao Y. Induction of apoptosis by epigallocatechin-3-gallate via mitochondrial signal transduction pathway. Prev Med. 2004;39:1172–1179. doi: 10.1016/j.ypmed.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Noguchi M, Nakao Y, Pater A, Iwasaka T. The tea polyphenol, (−)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol Oncol. 2004;92:197–204. doi: 10.1016/j.ygyno.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Chen JJ, Ye ZQ, Koo MW. Growth inhibition and cell cycle arrest effects of epigallocatechin gallate in the NBT-II bladder tumour cell line. BJU Int. 2004;93:1082–1086. doi: 10.1111/j.1464-410X.2004.04785.x. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 7.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10:219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 8.Fujita Y, Yamane T, Tanaka M, et al. Inhibitory effect of (−)-epigallocatechin gallate on carcinogenesis with N-ethyl-N′-nitro-N-nitrosoguanidine in mouse duodenum. Jpn J Cancer Res. 1989;80:503–505. doi: 10.1111/j.1349-7006.1989.tb01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komori A, Yatsunami J, Okabe S, et al. Anticarcinogenic activity of green tea polyphenols. Jpn J Clin Oncol. 1993;23:186–190. [PubMed] [Google Scholar]
- 10.Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 11.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 12.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem Biol Interact. 2008;171:89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Chung LY, Cheung TC, Kong SK, et al. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001;68:1207–1214. doi: 10.1016/s0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 16.Ravindranath MH, Ramasamy V, Moon S, Ruiz C, Muthugounder S. Differential growth suppression of human melanoma cells by tea (Camellia sinensis) epicatechins (ECG, EGC and EGCG) Evid Based Complement Alternat Med. 2009;6:523–530. doi: 10.1093/ecam/nem140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katdare M, Osborne MP, Telang NT. Inhibition of aberrant proliferation and induction of apoptosis in pre-neoplastic human mammary epithelial cells by natural phytochemicals. Oncol Rep. 1998;5:311–315. doi: 10.3892/or.5.2.311. [DOI] [PubMed] [Google Scholar]
- 18.Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]