Abstract
Background
Whether ApolipoproteinE (APOE) E4 allele status which is associated with an increased risk of cognitive decline is also associated with hearing impairment is unknown.
Methods
We studied 1833 men and women enrolled in the Health, Aging and Body Composition study. Regression models adjusted for demographic and cardiovascular risk factors were used to assess the cross-sectional association of APOE-E4 status with individual pure tone hearing thresholds and the 4-frequency pure tone average (0.5 kHz–4kHz) in the better hearing ear.
Results
Compared to participants with no APOE-E4 alleles, participants with one allele had better thresholds at 4.0kHz (β= −2.72dB, p = 0.013) and 8.0 kHz (β= −3.05kHz, p = 0.006), and participants with two alleles had better hearing thresholds at 1.0kHz (β= −8.56dB, p=0.021).
Conclusion
Our results suggest that APOE-E4 allele status may be marginally associated with better hearing thresholds in older adults.
Keywords: Apolipoprotein E, hearing thresholds, hearing loss, cognition, aging, dementia
INTRODUCTION
In epidemiologic studies, hearing loss in older adults has been found to be independently associated with the risk of cognitive decline,1 incident dementia,2,3 decline in physical function,4 gait speed,5 increase in falls,6 and smaller social network.7 Potential mechanistic pathways underlying this association include the effects of hearing loss on social isolation, brain structure, and/or cognitive load1. It is unknown whether ApolipoproteinE (APOE) E4 allele status which is strongly associated with an elevated risk of cognitive decline and dementia8 is also associated with hearing loss. Only two prior studies have investigated the association of APOE genotype, a 299 residue plasma protein existing as three alleles (E2, E3, and E4), with hearing thresholds, and these studies have produced conflicting results (Table1). The Leiden 85-plus study by Kurniawan et al. (2012) demonstrated that individuals homozygous for APOE-E4 (n=6) had poorer hearing than individuals with only one or zero APOE-E4 alleles.5 In contrast, O’Grady et al. (2007) demonstrated that the E4 allele was less likely to be observed in participants with sensorineural hearing loss than the general population.6 In this study, we analyze data on APOE-E4 status and audiometric hearing thresholds from participants followed in the Health, Aging and Body Composition (Health ABC) study to investigate whether the E4 allele is independently associated with hearing thresholds
Table 1.
Characteristics of prior studies investigating the association of hearing loss and APOE-E4 allele status.
| Cohort | Analytic Sample | Study Objective | Findingsa | |||
|---|---|---|---|---|---|---|
| Kurniawan et al. (2012) | Leiden 85 Plus Study | 435 | Hearing Thresholds by APOE-E allele status | E4 (−/−) 56.1 dB Loss (n=6) |
E4 (+/−) 51.0 dB Loss (n=89) |
E4(+/+) 48.9 dB Loss (n=340) |
| O’Grady et al. (2007) | Convenience Sample | 89 | Observed vs expected allele frequencies by severity of hearing loss | No association of E4 Allele and hearing loss (n=18 E4 alleles) | ||
E4 (−/−), Individuals with zero E4 alleles. E4 (−/+), individuals with one E4 allele. E4 (+/+), individuals with two E4 alleles
METHODS
Study Population
The Health, Aging and Body Composition (Health ABC) study is a prospective observational study that enrolled 3075 well-functioning, community-dwelling participants aged 70–79 years starting in 1997–1998. Study participants were recruited from a random sample of White Medicare beneficiaries and all eligible Black race/ethnicity living within a one-hour drive from the examination site in the cities of Pittsburgh, Pennsylvania and Memphis, Tennessee. Participants were excluded if they reported a history of active treatment for cancer in the previous 3 years, planned to move out of the study area within the next 3 years, or were currently participating in a lifestyle intervention trial. To be eligible, participants had to report no difficulty with walking a quarter mile, climbing 10 steps without resting, or performing basic activities of daily living. Race was restricted to White and Black individuals because one of the original study objectives was to examine race differences in body composition parameters, and there were insufficient resources to recruit additional races or ethnicities.
DNA testing for APOE was performed at study enrollment in year 1 (n=2909), and of those participants, 2089 individuals underwent audiometric testing at their year 5 follow-up. Various causes [e.g., attrition from death (n=263), refused/unable (n=89), no clinical visit in year 5 (n=437), other (n=31)] prevented some participants enrolled at baseline from receiving audiometric testing in year 5. We then excluded participants with missing covariate data (n=59) and individuals with a Modified Mini-Mental Status [3MS] < 80 (n=197). Our final analytic cohort was comprised of 1833 participants with complete data and no evidence of cognitive impairment defined by a Modified Mini-Mental State [3MS] ≥ 80. All study participants signed a written informed consent, and this study was approved by the institutional review boards at each respective study site.
Apolipoprotein E
Complete APOE genotypes were obtained using standard single nucleotide polymorphism techniques by Bioserve, Ltd. (Laurel, MD). Participants were defined as having no E4 alleles (homozygote E4 negative), one E4 allele (heterozygote E4 positive), and two E4 alleles (homozygote E4 positive) based on genotype.
Audiometry
Air-conduction pure tone hearing thresholds were obtained at octave frequencies from .25 through 8 kHz using standard audiometric testing procedures (ANSI, 1978). The testing was completed with an audiometer (Maico MA40) and supra-aural earphones (TDH 39) with the participant seated in a sound-attenuating booth and the examiner outside the booth. Both the audiometer and booth met prevailing ANSI standards for threshold testing. The thresholds were recorded in decibels hearing level (dB HL) and a 4-frequency pure tone average (PTA) of hearing thresholds obtained at 0.5, 1, 2 and 4 kHz was calculated for the better hearing ear.
Other Covariates
At enrollment, participants reported their age, sex, race, and educational history. Pre-specified algorithms based on both self-report and physician diagnoses, recorded medications, and laboratory data were used to define the presence of hypertension (based on clinic measure, medications, or self-report) and diabetes mellitus (based on fasting blood glucose level, medications, or self-report) in year 5 of the study. Stroke history and smoking status (current/former/never) were based on interviewer-administered questionnaires. Serum fasting total cholesterol (mg/dL) was measured in year 1 on a commercially available analyzer (Vitrox 950, Johnson & Johnson).
Statistical Analyses
All participants were categorized according to the number of APOE-E4 alleles in their genotype, and those with no APOE-E4 alleles were considered the reference group for all comparisons. Associations between APOE status and continuous covariates were explored using the Kruskal Wallis test, and associations with categorical covariates were explored using Fisher’s Exact test. Observed allele frequencies were compared to established population allele frequencies using a chi-square test. Average, adjusted hearing thresholds across 0.5 kHz – 8 kHz range were modeled using profile analysis with an unstructured covariance matrix. These frequencies were selected because they are considered the most important for spoken language (0.5 khz–4 khz), with the addition of 1 high frequency known to have variance in older persons (8 khz). Profile analysis models were used to create covariate-adjusted mean audiograms for each group. Multiple linear regression using robust regression models were used to investigate the association of APOE status and the 4-frequency PTA in the better hearing ear. Profile analysis and multiple linear regression models were adjusted for age, demographic (sex, education, and race), and cardiovascular risk factors (fasting total cholesterol at baseline and smoking status and history of hypertension, diabetes and stroke). Sensitivity analyses were then performed with results stratified by race and sex, demographic characteristics strongly associated with hearing loss. Regression model assumptions were verified using diagnostic plots. Significance testing for all analyses was conducted using 2-sided tests with a type I error of 0.05. All analyses were conducted using SAS, version 9.2 (SAS Institute Inc. Cary, North Carolina).
RESULTS
At baseline, participants with two APOE-E4 alleles were more likely to be younger, black, have post-secondary education, and have higher total fasting cholesterol levels compared to participants with zero or one APOE-E4 allele (Table 2). Observed and expected APOE allele frequency distributions in participants did not significantly differ from established general population allele frequency distributions (Table 3).
Table 2.
Demographic and clinical characteristics of baseline (Year 5) study cohort by apolipoprotein E allele status. Health, Aging and Body Composition Study (n=1833).
| Characteristic | E4 (−/−)a | E4 (+/−)a | E4(+/+)a | P Value |
|---|---|---|---|---|
| n=1340 | n=470 | n=23 | ||
| Site | ||||
| Memphis | 598 (44.6) | 228 (48.5) | 10 (43.5) | .34 |
| Pittsburgh | 742 (55.4) | 242 (51.5) | 13 (56.5) | |
| Female | 687 (51.3) | 257 (54.7) | 11 (47.8) | .39 |
| Age, Mean (S.D.), y | 77.5 (2.9) | 77.1 (2.7) | 76.5 (2.5) | .02 |
| Race | ||||
| White | 970 (72.4) | 276 (58.7) | 12 (52.2) | |
| Black | 370 (27.6) | 194 (41.3) | 11 (47.8) | <.001 |
| Diabetes | 241 (18.0) | 84 (17.9) | 3 (13.0) | .92 |
| Smoking | ||||
| Current | 73 (5.5) | 28 (6.0) | 0 (0) | .89 |
| Former | 667 (49.8) | 230 (48.9) | 11 (47.8) | |
| Never | 600 (44.8) | 212 (45.1) | 12 (52.2) | |
| Hypertension | 1043 (77.8) | 356 (75.7) | 18 (78.3) | .67 |
| Stroke | 113 (8.4) | 40 (8.5) | 0 (0) | .45 |
| Education | ||||
| Less than High School | 211 (15.8) | 93 (19.8) | 1 (4.4) | .03 |
| High School | 451 (33.7) | 173 (36.8) | 8 (34.8) | |
| Post-Secondary | 678 (50.6) | 204 (43.4) | 14 (60.9) | |
| Total Fasting Cholesterol (S.D.), mg/dl Year 1 | 202.3 (38.0) | 207.0 (38.9) | 215.7 (49.7) | .02 |
Abbreviations: S.D., standard deviation. ll values are expressed as No. (%) of participants unless otherwise indicated.
E4 (−/−), Individuals with zero E4 alleles. E4 (−/+), individuals with one E4 allele. E4 (+/+), individuals with two E4 alleles
Table 3.
Observed and expected APOE allele frequency in participants compared to general population.b
| E2 | E3 | E4 | P value | |
|---|---|---|---|---|
| Observed a | 322 (0.09) | 2828 (0.77) | 516 (0.14) | .086 |
| Expected b | 293.3 (0.08) | 2822.8 (0.77) | 549.9 (0.15) |
All values are expressed as No. (%) of participants.
Observed frequencies may not equal 100% due to rounding.
General population established allele frequencies of 0.08 for E2, 0.77 for E3, and 0.15 for E49
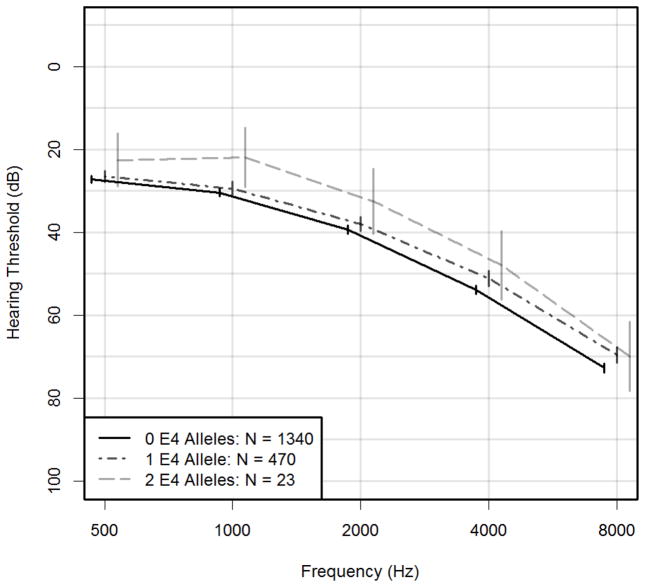
Mean hearing thresholds in the better ear at each pure tone frequency according to APOE-E4 allele status were compared using mixed effects models (Figure 1). Compared to participants with no E4 alleles, participants with one allele had better thresholds at 4.0 kHz (β = −2.72 dB, p = 0.013) and 8.0 kHz (β =−3.05 kHz, p = 0.006), and participants with two alleles had better hearing thresholds at 1.0 kHz (β = −8.56 dB, p =0.021) (Table 4). Results stratified by race and sex demonstrated similar results with greater number of E4 alleles generally being associated with marginally better hearing thresholds (Table 4).
Figure 1.
Better Ear Mean Audiograms by E4 allele status. Error bars correspond to 95% confidence intervals. Adjusted for age, sex, race, education, study site, diabetes, smoking status, hypertension, stroke, and total fasting cholesterol.
Table 4.
Association of APOE-E4 allele status with each pure tone hearing threshold.a
| Better ear pure tone hearing threshold Beta (95% CI) | |||||
|---|---|---|---|---|---|
| 0.5 kHz | 1.0 kHz | 2.0 kHz | 4.0 kHz | 8.0 kHz | |
| All Participants (N= 1833) | |||||
|
E4 (−/−, n=)b N= 1340 |
Ref | Ref | Ref | Ref | Ref |
|
E4 (+/−)b N= 470 |
−0.64(−2.28, 1.01) | −1.05(−2.91, 0.81) | −1.35(−3.38, 0.68) | −2.72(−4.85, −0.58)* | −3.05(−5.22, −0.87)** |
|
E4(+/+)b N=23 |
−4.65(−11.07,1.77) | −8.56(−15.80, −1.32)* | −6.77(−14.71,1.16) | −5.90(−14.24,2.45) | −2.81 (−11.31, 5.69) |
|
| |||||
| Black (N=575) | |||||
|
E4 (−/−)b N=370 |
Ref | Ref | Ref | Ref | Ref |
|
E4 (+/−)b N=194 |
−2.65(−5.32, 0.01) | −2.98(−5.87, −0.09)* | −2.53(−5.59, 0.53) | −3.72(−7.09, −0.35)* | −3.61(−7.39, 0.17) |
|
E4(+/+)b N=11 |
−7.33(−16.55, 1.88) | −9.53(−19.52, 0.45) | −10.85(−21.43, −0.28)* | −10.08(−21.73,1.57) | −6.67 (−19.72, 6.38) |
|
| |||||
| White (N=1258) | |||||
|
E4 (−/−)b N=970 |
Ref | Ref | Ref | Ref | Ref |
|
E4 (+/−)b N=276 |
−0.57(−2.66, 1.51) | −1.00 (−3.40, 1.40) | −0.87 (−3.54,1.80) | −0.65(−3.34, 2.04) | −1.58(−4.23, 1.06) |
|
E4(+/+)b N=12 |
−3.26 (−12.11, 5.60) | −8.65 (−18.86, 1.56) | −2.41 (−13.77, 8.95) | 1.41(−10.03, 12.86) | 3.72 (−7.52, 14.97) |
|
| |||||
| Men (N=878) | |||||
|
E4 (−/−, n=)b N= 653 |
Ref | Ref | Ref | Ref | Ref |
|
E4 (+/−)b N= 213 |
−1.14(−3.58, 1.31) | −0.72(−3.60, 2.16) | −0.56(−3.81, 2.70) | −1.01(−4.05, 2.03) | −1.94(−5.07,1.19) |
|
E4(+/+)b N=12 |
−2.74(−11.74, 6.27) | −9.06(−19.68, 1.56) | −6.24(−18.24, 5.75) | −0.77(−11.98, 10.44) | 2.45 (−9.10, 13.99) |
|
| |||||
| Women (N=955) | |||||
|
E4 (−/−, n=)b N= 687 |
Ref | Ref | Ref | Ref | Ref |
|
E4 (+/−)b N= 257 |
−0.87(−3.03, 1.29) | −1.80(−4.20, 0.60) | −2.09 (−4.59, 0.42) | −3.66(−6.36, −0.97)** | −4.10(−7.12, −1.09)** |
|
E4(+/+) N=11 |
−6.12(−15.00, −2.75) | −7.59(−17.49, 2.31) | −7.40 (−17.73, 2.94) | −12.17(−23.31, −1.03)* | −8.53 (−20.99, 3.94) |
Abbreviations: Ref., reference.
p<0.05;
p<.01 compared to individuals with zero E4 alleles.
Adjusted for age, sex, race, education, study site, diabetes, smoking status, hypertension, stroke, and total fasting cholesterol.
E4 (−/−), Individuals with zero E4 alleles. E4 (-/+), individuals with one E4 allele. E4 (+/+), individuals with two E4 alleles Negative values indicate better hearing thresholds compared to individuals with zero E4 alleles (reference).
Analyses using the 4-frequency pure tone average demonstrated no significant differences in the average PTA in individuals with one allele (β = −0.32 dB, 95% CI: −[1.61 dB, 0.9 dB], p=0.63) or two alleles (β = −3.53 dB, 95% CI:[ −8.54 dB, 1.50 dB], p=0.17) compared to participants with zero E4 alleles in fully-adjusted models. Analyses stratified by race and sex also demonstrated similar findings (data not shown), except that among black participants, those with two E4 alleles had better average PTA compared to participants with zero E4 alleles (β = −7.06 dB, 95% CI: − [13.76 dB, −0.36 dB], p=0.04). Finally, we conducted a sensitivity analysis to investigate if excluding individuals with cognitive impairment at year 5 (3MS score < 80) may have biased our results. In this analysis that included all participants regardless of 3MS score in Year 5 (n= 2030), our findings were substantively unchanged (mean PTA difference compared to zero E4 alleles [n = 1452]: one allele [n = 544] β = −0.54, 95% CI:[ −1.76, 0.68], p = 0.39; two alleles [n = 34] β = −3.83, 95% CI:[ −8.00, 0.33], p = 0.07).
DISCUSSION
Our findings suggest that APOE-E4 allele status may be marginally associated with better hearing thresholds in older adults. Compared to individuals with zero APOE-E4 alleles, those individuals with one or two APOE-E4 alleles generally had slightly better hearing thresholds in a dose-dependent manner, though many comparisons did not reach significance. These results must be interpreted with caution, however, given that few participants (n=23, 1.3%) were homozygous for APOE-E4. Overall, these findings indicate that APOE-E4 status may be associated with better hearing function among older adults without cognitive impairment and indirectly suggests that APOE-E4 allele status would be unlikely to appreciably confound the association of hearing loss with impaired cognitive functioning that has been observed in prior epidemiologic studies.
Two previous studies investigated the association of APOE-E4 allele status with hearing thresholds. The Leiden 85-plus study by Kurniawan et al. (2012) demonstrated that individuals homozygous for APOE-E4 had poorer hearing (mean 56.1 dB PTA at 1, 2, and 4 kHz) than individuals with only one (mean 51.0 dB PTA) or zero (mean 48.9 dB PTA) APOE-E4 alleles.10 However, this association was primarily driven by a very small number of participants with two APOE-E4 alleles (n=6) and, therefore, should be interpreted with caution. Another possible limitation of the Kurniawan et al (2012) study10 is that hearing testing was performed during home visits with a portable audiometer without a sound attenuating booth. Hearing thresholds gathered under such conditions can markedly vary depending on the level of ambient noise and the testing environment which would likely be different from household to household. In another study with conflicting results, O’Grady et al. (2007) demonstrated in a convenience sample of outpatient clinic participants that the APOE-E4 allele was less likely to be observed in participants with sensorineural hearing loss than the general population.11 However, these results also were based on a small sample of individuals (n=89).
Our results suggest a weak protective association between APOE-E4 allele status and hearing thresholds in the mid to high frequencies in older adults. One explanation for this finding is that there was a higher proportion of black participants having at least one APOE-E4 allele, and the odds of hearing loss have been observed to be substantially lower in black individuals (possibly because of a protective effect of melanin in the cochlea).12 Individuals with two APOE-E4 alleles in our cohort also may reflect healthy survivors with better overall health and hence better hearing thresholds. Indeed, these individuals had a lower prevalence of smoking and stroke, and higher education levels than participants with zero or one APOE-E4 allele. Although we accounted for these factors in our analyses through adjustment or stratification, we are unable to exclude the possibility of residual confounding as potentially underlying the protective association observed between APOE-E4 and hearing thresholds. A plausible biological mechanism through which APOE-E4 allele status would promote better auditory function in the cochlea is unknown. Overall, we believe that the contribution of the APOE-E4 allele to better hearing thresholds in older adults is likely to be very modest at best.
Strengths of our study include the availability of a relatively large cohort of older adults who had audiometric assessments performed under standardized conditions in a sound attenuating booth and the ability to account for multiple potential confounders and effect modifiers in our analyses. The primary study limitation is the relatively few participants with two APOE-E4 alleles (n=23, 1.3%) versus approximately 2.2% in the general population9 and hence our results may not be generalizable. One explanation may be that well-functioning community individuals were recruited for study participation, thus possibly excluding individuals with two APOE-E4 alleles who may be predisposed to early onset dementia and other health issues. Hearing thresholds also were measured only once and therefore, we could not estimate the potential association between APOE-E4 allele status and trajectories of hearing decline. Finally, we had no additional information on the possible etiology of hearing loss for study participants. However, we believe that it is unlikely that these limitations would substantially bias our findings.
In summary, our results suggest that APOE-E4 allele status may be weakly associated with better hearing thresholds in older adults. Future investigations in cohort studies with longitudinal data on hearing thresholds will allow for a better understanding of how APOE-E4 allele status may be associated with declines in hearing function over time.
Acknowledgments
Funding: Dr. Lin was supported by a grant from the National Institute On Deafness and Other Communication Disorders (K23DC011279), by the Triological Society/American College of Surgeons Clinician Scientist Award, and the Eleanor Schwartz Charitable Foundation. This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Disclosures: All authors contributed to the study concept and design, analysis and interpretation of data, and preparation of the final manuscript.
Conflicts of Interest Disclosure: Dr. Lin reports being a consultant to Pfizer, Cochlear Corp, & Autifony, serves on the scientific advisory board for Autifony, and has been a speaker for Amplifon & Cochlear Corp. Sheila Pratt was supported with resources and the use of facilities at the VA Pittsburgh Healthcare System, Pittsburgh, PA.
References
- 1.Lin FR, Yaffe K, Xia J, et al. Hearing Loss and cognitive decline in older adults. JAMA Internal Medicine. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 3.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurology. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loprinzi PD, Smit E, Lin FR, Gilham B, Ramulu PY. Accelerometer-assessed physical activity and objectively determine dual sensory impairment in US adults. [published online ahead of print June 7 2013] [accessed June 10, 2013.];May Clin Proc. 2013 doi: 10.1016/j.mayocp.2013.04.008. http://www.mayoclinicproceedings.org/current. [DOI] [PubMed]
- 5.Li L, Simonsick EM, Ferrucci L, Lin FR. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38(1):25–29. doi: 10.1016/j.gaitpost.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer SE, Kapteyn TS, Kuik DJ, Deeg DJH. The association of hearing impairment and chronic diseases with psychosocial health status in older age. Journal of Aging and Health. 2002;14(1):122–137. doi: 10.1177/089826430201400107. [DOI] [PubMed] [Google Scholar]
- 8.Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systemic Review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153(3):182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 9.Menzel HJ, Kladetzky RG, Assmann G. Apolipoprotein polymorphism and coronary artery disease. Arteriosclerosis 1983. 1983;983:310–315. doi: 10.1161/01.atv.3.4.310. [DOI] [PubMed] [Google Scholar]
- 10.Kurniawan C, Westendrop RGJ, de Craen AJM, et al. Gene dose of apolipoprotein E and age-related hearing loss. Neurobiology of Aging. 2012;33:2230.e7–e12. doi: 10.1016/j.neurobiolaging.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.O’Grady G, Boyles AL, Speer M, DeRuyter F, Strittmatter W, Worley G. Apolipoprotein E alleles and sensorineural hearing loss. International Journal of Audiology. 2007;46:183–186. doi: 10.1080/14992020601145294. [DOI] [PubMed] [Google Scholar]
- 12.Lin FR, Maas P, Chien W, Carey JP, Ferrucci L, Thorpe R. Association of skin color, race/ehtnicity, and hearing loss among adults in the USA. 2012;13(1):109–117. doi: 10.1007/s10162-011-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]