Abstract
Alzheimer’s disease (AD) affects millions of people worldwide. The neuropathologic process underlying AD begins years, if not decades, before the onset of memory decline. Recent advances in neuroimaging suggest that it is now possible to detect AD-associated neuropathological changes well before dementia onset. Here, we evaluate the role of recently developed in vivo biomarkers in the clinical evaluation of AD. We discuss how assessment strategies might incorporate neuroimaging markers to better inform patients, families and clinicians when memory impairment prompts a search for diagnosis and management options.
AD pathobiology
Since their first description by Alois Alzheimer in 1907, 3 amyloid-containing plaques and tau-associated neurofibrillary tangles (NFTs) have remained the two hallmark pathological lesions of AD. Senile and neuritic plaques are composed of amyloid-beta (Aβ), a 38–43 amino acid peptide that derives from the much larger cell membrane-associated amyloid precursor protein and gradually accumulates over time in the extracellular spaces of the brain. 4 Within plaques, Aβ is present in aggregated/insoluble forms such as fibrils and soluble forms such as oligomers. 5 In animal models, Aβ initiates downstream loss of dendrites and synapses 5 and functional disruption of neuronal networks.6 Genetic evidence indicates that APOE ε4, the most important known genetic risk factor for late onset AD, accelerates the onset of Aβ deposition into plaques and decreases the transport of Aβ across the blood brain barrier. 7 Furthermore, a recently discovered mutation in Aβ-precursor protein protects against AD 8 providing additional evidence regarding the central role of Aβ in AD pathogenesis. However, neocortical Aβ plaques are present not only in cognitively impaired patients but also in cognitively normal older adults. 9 Poor correlations between Aβ deposition and memory decline, 10 together with the observation that immunotherapy-induced Aβ plaque removal may not prevent neurodegeneration, 11 suggest that additional entities besides Aβ are required for AD-associated degeneration.
Neurofibrillary tangles, primarily found in neuronal cell bodies, are composed of the hyperphosphorylated, aggregated form of the microtubule binding protein, tau. Unlike Aβ plaques, tau-associated NFTs strongly correlate with clinical severity 10 and follow a defined temporal topographic pattern in which medial temporal lobe regions underlying memory function are affected in the earliest stages of the disease. 12 Recent work in animal models 13,14 and humans 15,16 points to a synergistic relationship between Aβ and tau whereby Aβ-associated neurodegeneration occurs only in the presence of tau. Intriguingly, evidence from animal models indicates that reducing tau levels rescues mice from premature mortality and memory deficits without altering Aβ levels or plaque burden. 13 These findings, along with other biochemical and experimental evidence, support a two-stage disease process in which Aβ deposition initiates the neurodegenerative cascade (including tau hyperphosphorylation and aggregation), which in turn becomes increasingly independent of the initiating Aβ. 17
Imaging and fluid biomarkers for assessing Alzheimer’s pathology
Neuropathological findings indicate that Aβ accumulation and tau pathology begin years or even decades before the onset of clinical symptoms. 18 Neuroimaging and cerebrospinal fluid (CSF) markers can detect the earliest pathologic changes associated with AD enabling identification of clinically normal individuals in the pre-symptomatic or pre-clinical stage of AD. 19 In the sections below, we review the most extensively validated in vivo biomarkers of amyloid pathology and AD-related neurodegeneration. For simplicity, we do not review the putative markers of synaptic injury, such as FDG-PET or functional MRI, which may prove useful in distinguishing between certain neurodegenerative disorders.
Volumetric structural MRI
Structural MRI is a convenient first imaging modality for assessing AD neurodegeneration because current practice guidelines include its use during the routine evaluation of patients with cognitive complaints, primarily to exclude structural abnormalities such as infarction, brain tumors or hydrocephalus. 20 Brain atrophy on structural MRI reflects the loss of dendrites, synapses, and neurons. 21 Though atrophy is not specific to AD, there is a strong association between the severity of atrophy and cognitive decline along the aging continuum and the degree of atrophy correlates with Braak pathologic staging at autopsy. 21 Importantly, the topographic distribution of MRI-based atrophy in AD maps well onto the distribution of NFT pathology, with the entorhinal cortex and hippocampus demonstrating the largest magnitude of gray matter loss in patients with a high tau burden. 22
A number of methodologies, ranging from whole brain or voxel-based techniques to region of interest-based methods, have been proposed to quantitatively evaluate brain atrophy on MRI. Within the last decade, the routine acquisition of high-quality 3D T1-weighted images and rapid advances in image analysis algorithms have led to the availability of volumetric MRI-based software tools (vMRI) capable of automatically subdividing the brain into neuroanatomic regions and quantifying tissue loss within each region for a single individual. 23–25 Fully automated quantitative vMRI-based neuroanatomic assessments can detect AD-associated volume loss, predict disease progression, and be used as an outcome measure in therapeutic trials. 26,27 Recently, the FDA has approved one such vMRI technology 28 that can assist in the clinical work-up of memory decline (Figure 1). In Table 2, we review recent (from 2009–2012) prospective studies using vMRI methods to predict clinical progression from MCI to AD.
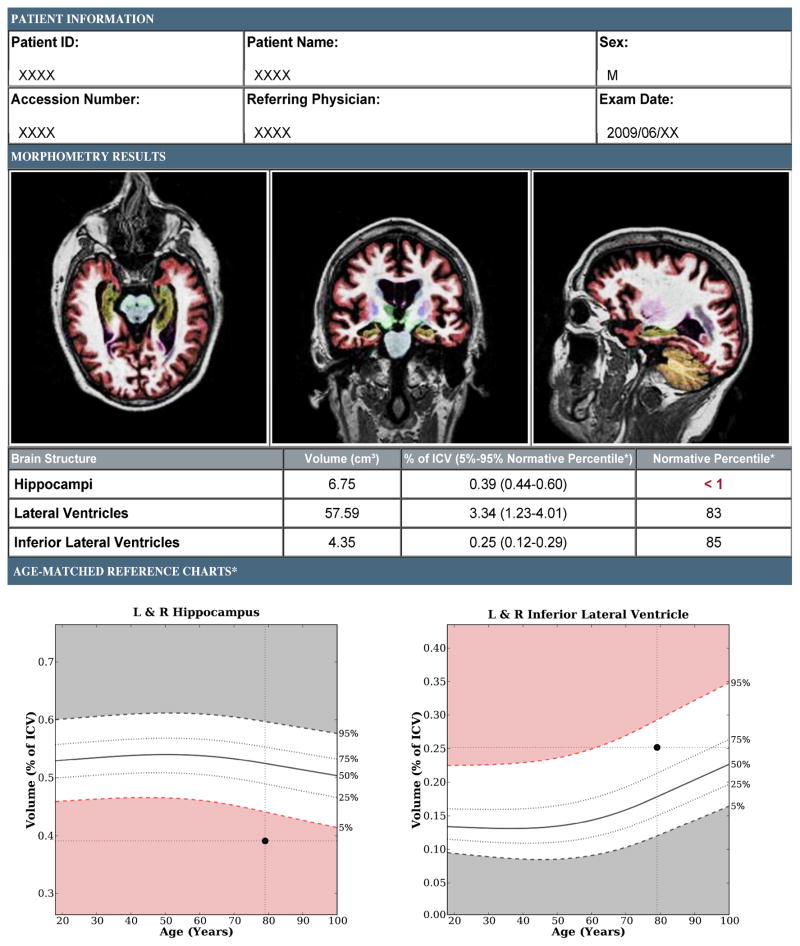
Figure 1.
Brain MRI evaluation of a patient with amnestic MCI using a volumetric technique (NeuroQuant™, http://www.cortechslabs.com). The top panel illustrates subcortical regions, such as the hippocampus (shown in dark yellow), automatically classified on axial, coronal, and sagittal T1-weighted MRI images. The middle and bottom panel demonstrate volumes and normative percentiles for the hippocampus and ventricles. Analyses of the baseline MRI scan demonstrated hippocampal volumes that were at the < 1 normative percentile, lending objective support to an impression of medial temporal lobe atrophy. At the time of volumetric assessment, the patient’s Mini-Mental Status Exam (MMSE) was 29/30 yet memory impairment was suggested by more detailed neuropsychological testing. Three years later, his MMSE was 22/30 and he had clinically progressed to dementia with high biomarker probability of AD, as supported by evidence of neuronal injury on structural MRI and elevated amyloid on a florbetapir scan (see Figure 3).
Table 2.
Studies in non-demented older individuals evaluating the ability of automated, volumetric MRI (vMRI) studies for predicting the risk of progressing to AD. Given the vast number of studies evaluating the ability of MRI measures to predict progression from MCI to AD, we focused on prospective studies using automated or semi-automated MRI methods and provide representative examples. We assessed the levels of evidence using the AHA/ASA guidelines. 50 ADNI = a multi-site, multi-center cohort from North America, AddNeuroMed = a multi-site, multi-center cohort from Europe.
| Study | Participants | Source of Participants | Study Quality Level | vMRI Method | Results | Limitations |
|---|---|---|---|---|---|---|
| Bakkour et al., 200951 | 49 cognitively impaired older adults | Memory disorders clinic from the US | B# | Baseline regional gray matter thickness | Predicted AD progression with 85% sensitivity and 65% specificity | Hippocampus and ventricles not assessed |
| Costafreda et al., 201152 | 103 MCI patients | AddNeuroMed Consortium | B | Hippocampal shape analysis | Predicted AD progression at 1 year with 77% sensitivity and 80% specificity | Clinical applicability to population based cohort not assessed |
| Den Heijer et al., 201053 | 518 older adults | Population based cohort from the Netherlands | B | Hippocampal volume | Baseline (mean HR = 2.2) and longitudinal (mean HR = 1.6) hippocampal volumes associated with higher dementia risk | Manual correction of brain regions was necessary in a subset of cases |
| Desikan et al., 200954 | 129 cognitively impaired older adults | Population based cohort primarily from East Boston | B | Baseline regional gray matter volumes | Combination of entorhinal cortex (HR = 0.60) and inferior parietal lobule (HR = 0.62) best predicted time to AD progression | Manual correction of brain regions was necessary in a subset of cases |
| Heister et al., 201155 | 192 MCI patients | ADNI | B | Fully-automated baseline hippocampal occupancy score | Predicted time to AD progression (HR = 3.9) | Clinical applicability to population based cohort not assessed |
| Jack et al., 201056 | 218 MCI patients | ADNI | B | Baseline hippocampal volume with high (75th percentile) and low (25th percentile) amyloid deposition | Predicted time to AD progression (HR = 2.6) | Clinical applicability to population based cohort not assessed |
| Kovacevic et al., 200957 | 269 MCI patients | ADNI | B | Fully-automated baseline volumes of medial temporal lobe | Smaller brain associated with longitudinal decline in MMSE and CDR-SB | Clinical applicability to population based cohort not assessed |
| Sluimer et al., 200958 | 44 MCI patients | Memory disorders clinic from the Netherlands | C | Longitudinal atrophy rates of six brain regions | Medial temporal lobe atrophy best predicted (HR = 15.8) time to AD progression | Very limited populations examined |
| Vemuri et al., 200959 | 192 MCI patients | ADNI | B | Structural abnormality index score (STAND) | Predicted time to AD progression (HR = 2.6, 75th versus 25th percentile) | Unclear clinical applicability of vMRI method |
| Westman et al., 201160 | 101 MCI patients | AddNeuroMed Consortium | B | Hippocampal volume and gray matter thickness | Correctly classified 74% of MCI patients who progressed to AD at one year | Clinical applicability to population based cohort not assessed |
However, structural MRI has limitations. vMRI does not directly evaluate Aβ and tau but rather provides an indirect assessment of neurodegeneration that occurs downstream from molecular pathology. Another limitation is that though certain patterns of volume loss are characteristic of different diseases (e.g. entorhinal cortex atrophy in AD), the finding of medial temporal lobe atrophy by itself is nonspecific and can also be seen in other neurologic and psychiatric disorders. Therefore, vMRI of medial temporal lobe structures, in isolation, cannot distinguish AD from hippocampal sclerosis or other neurodegenerative diseases such as frontotemporal dementia (FTD). Moreover, neuropathologic evidence demonstrates the presence of uncommon AD subtypes that spare the medial temporal lobes, especially in younger patients. 29 Despite these weaknesses, given its capability for precise anatomic description with high reliability, analysis of MRI data across a wide range of scanner types/manufacturers and the ability to efficiently generate normative databases from multi-center data, vMRI will undoubtedly play a significant role in decision-making during the clinical evaluation of dementia. The optimal diagnostic and prognostic value of vMRI will be obtained when combined with clinical/cognitive testing and other markers including CSF and imaging measures of Alzheimer’s pathology.
Molecular imaging and fluid biomarkers of Aβ deposition
Within the last decade, a number of PET-based radiotracers have been developed to noninvasively assess for the presence of Aβ, of which the most extensively examined is 11C-labelled [N-methyl]-2-(4′-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburgh Compound-B, PIB). Studies using transgenic mouse models and human brain sections indicate that PIB selectively binds to the fibrillar form of Aβ in neuritic plaques and cerebral amyloid angiopathy. 30,31 In vivo, ante-mortem PIB retention strongly correlates with in vitro, post-mortem measures of fibrillar Aβ pathology in autopsy-confirmed AD but does not associate with NFTs, Lewy bodies or other protein aggregates. 32,33 In humans, the overall pattern of increased PIB retention mirrors the distribution of fibrillar Aβ plaques found at autopsy and involves the prefrontal, parietal, and lateral temporal cortices. 34 A recent review suggests that the overwhelming majority of AD patients and cognitively impaired individuals who progress to AD are amyloid ‘positive’. 35 Furthermore, approximately 24% of cognitively normal older adults greater than 60 years of age also show increased cerebral PIB retention and the prevalence of amyloid positivity is closely related to age and APOE ε4 carrier status. 35 Together, these findings raise the possibility that amyloid imaging may yield positive results long before the appearance of cognitive symptoms, which, as discussed below, has both positive and negative consequences.
As either an alternative or adjunct to amyloid PET imaging, CSF sampling can also detect Aβ pathology. Though the majority of Aβ is produced in the brain and secreted into the extracellular spaces of the brain, a fraction of central nervous system-produced Aβ diffuses into the CSF and is present in modest concentrations (approximately 10–15 ng/mL). 36 CSF assessments measure the monomeric form of Aβ. Low CSF Aβ levels strongly correlate with increased PIB binding, intracranial plaque deposition and total Aβ load, demonstrating the value of these CSF measurements as a marker of fibrillar Aβ pathology. 36 However, an important clinical consideration with CSF sampling is the need for lumbar puncture, an uncomfortable procedure that carries a small risk of morbidity.
Imaging evaluation strategy for MCI and AD
Clinical assessment of the elderly patient with a memory complaint usually begins with a mental status evaluation to objectively confirm the presence of the cognitive deficit. If the degree of cognitive decline is greater than expected for healthy aging and further information is needed to guide management, the determination of whether a neurodegenerative process underlies the cognitive complaint can help determine which further diagnostic method to use. Above and beyond excluding other conditions to explain cognitive deficits (e.g. brain tumor, traumatic brain injury, infarctions, chronic hemorrhage, hydrocephalus, or encephalitis) structural MRI using vMRI techniques at this stage can be useful to document objective evidence of atrophy. As illustrated in Figure 2, vMRI can assist in supporting or contradicting a putative clinical diagnosis while providing an informative assessment of disease risk. The presence of reduced hippocampal volume provides support to the clinical impression that a neurodegenerative process contributes to the cognitive deficit but does not exclude the possibility of a congenitally small hippocampus or hippocampal damage from a prior insult. Importantly, low hippocampal volumes do not specify whether the underlying etiology is due to AD or other diseases such as frontotemporal dementia (FTD), dementia with Lewy bodies, or hippocampal sclerosis (Figure 2). Nevertheless, once a neurodegenerative etiology is supported through clinical and radiological evaluation, distinguishing among neurodegenerative disorders may benefit from supplemental testing for amyloid. This would likely be reserved for cases where additional tailoring of education or management is required and may be limited, as the more clinically relevant distinction is between benign or curable etiologies versus those with a near-term dire prognosis (Figure 2). It is important to note that in light of prior 11 and recent 37 clinical trials evidence that removing Aβ plaques using immunotherapeutic methods may not halt the neurodegenerative process, amyloid testing to confirm Alzheimer’s as the underlying etiology may prove most useful when therapies preventing downstream neurodegeneration become clinically available (Figure 2).
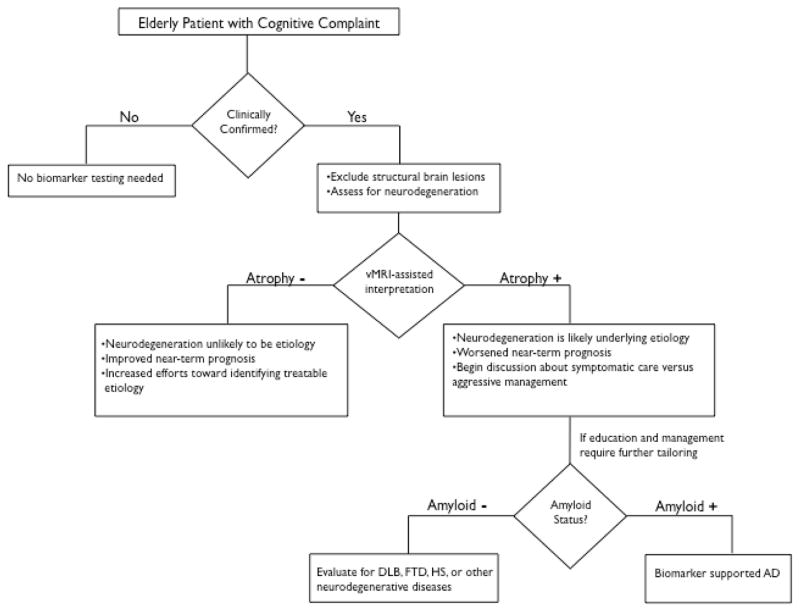
Figure 2.
Recommended decision tree for evaluating the elderly patient with a cognitive complaint. DLB = Dementia with Lewy bodies, FTD = frontotemporal dementia, HS = Hippocampal sclerosis, vMRI = automated volumetric MRI. Figure adapted from reference 63.
Challenges remain regarding the clinical application of vMRI in the patient with cognitive impairment. The difficulty in establishing normative ranges across a broad population of patients is a significant obstacle, but one that can be overcome by the availability of large databases of images in cognitively normal elders and subjects with both MCI and AD enrolled in multi-site, multi-national initiatives such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) in North America and the AddNeuroMed Consortium in Europe (see Table 2). Because atrophy is not diagnostic of AD neuropathologically and because the hippocampus is affected by a broad array of disorders, the diagnosis of AD cannot rely on simple ‘cut points’ or ‘thresholds’ in hippocampal volume 38, 39 derived from studies of progression to AD dementia. Furthermore, the degree of abnormality, along with other radiological features, including ex-vacuo dilatation of adjacent temporal horn and qualitative assessment of sulcal widening and cortical volume loss, will inform the impression of presence or absence of neurodegeneration. Importantly, the diagnosis of AD cannot be established by imaging alone; radiologic input serves to inform, rather than establish, an overall clinical impression.
Recommendations to use medial temporal atrophy on structural MRI among cognitively impaired individuals have already been proposed by an international AD working group40 and vMRI is one of the biomarkers recently incorporated into revised diagnostic criteria for AD, which noted that such biomarkers could serve “as optional clinical tools for use where available and when deemed appropriate by the clinician.” 41 Consistent with these recently revised diagnostic guidelines for AD 41 and MCI 42, by supporting the presence or absence of neurodegeneration, vMRI-based methods can also inform the likelihood of whether a patient with clinically confirmed memory loss will progress to dementia. The absence of vMRI-based brain atrophy diminishes the likelihood of neurodegeneration and increases the likelihood that a non-neurodegenerative, and potentially treatable, cause underlies the memory complaint. It is important to note that normal brain volumes for age, though not excluding the possibility of future neurodegeneration, can also be helpful for guiding clinical management while providing a more accurate predictive prognosis. Normal hippocampal volumes confer a better near-term prognosis and can foster increased efforts towards finding a treatable cause for the memory impairment while providing needed, albeit cautious, reassurance to the patient and caregivers who will be anxious about being given a dire prognosis.
Amyloid biomarkers in MCI and AD
The ability to specifically assess fibrillar Aβ pathology in vivo has generated considerable clinical excitement. Recently, the FDA has approved the fluorine-based amyloid tracer [F-18]florbetapir (Amyvid, Eli Lilly Inc) for use in patients being evaluated for AD and other causes of cognitive decline (Figure 3). Furthermore, commercial CSF Aβ assays with established normative ranges for amyloid status are now clinically available (http://www.athenadiagnostics.com). However, as noted by the FDA, though a negative florbetapir (amyloid) scan is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition, a positive florbetapir scan does not establish a diagnosis of AD. 43 Furthermore, elevated deposition of amyloid may occur in other neurological conditions and is often present in healthy elders with normal cognition. Recently, it has become increasingly evident that Aβ oligomers (e.g. dimers, trimers, tetramers and higher oligomers), rather than fibrillar Aβ plaques, represent the principal synaptotoxic form of amyloid that initiate the neurodegenerative process underlying AD. Insoluble Aβ fibrils, though serving as a reservoir for the neurotoxic oligomers, might themselves be relatively inactive. 44 Importantly, neither CSF analytes nor amyloid imaging can detect the oligomeric form of Aβ. 36 Similarly, in cognitively normal older individuals, though some studies have found a relationship between Aβ plaque deposition and neurodegeneration, 45,46 recent studies suggest that tau and other ‘downstream’ markers of neuronal injury modulate the effect of Aβ on cognitive decline and brain atrophy. 15–16, 47 In addition, recent clinical trials using monoclonal antibodies (solanezumab and bapineuzumab) that target Aβ and promote its clearance from the brain demonstrate minimal effect on disease trajectory modification in patients with mild or moderate AD: solanezumab showed marginal improvement in cognitive and functional decline and bapineuzumab, though affecting fibrillar Aβ and tau levels, did not modify the disease trajectory. 37 Taken collectively, this indicates that Aβ deposition precedes neurodegeneration and in the absence of cognitive decline or brain atrophy, represents an elevated risk state in much the same fashion that hypercholesterolemia serves as a risk factor for heart disease in the absence of myocardial damage. Just as cholesterol levels would not be used to diagnose a myocardial infarction in the setting of chest pain, detecting amyloid deposition may be less valuable than markers of neuronal damage when determining the cause of ongoing memory impairment. Nevertheless, it is hoped that a future contribution of Aβ testing, from diagnostic and therapeutic perspectives, may be among cognitively normal adults before the onset of neurodegeneration.
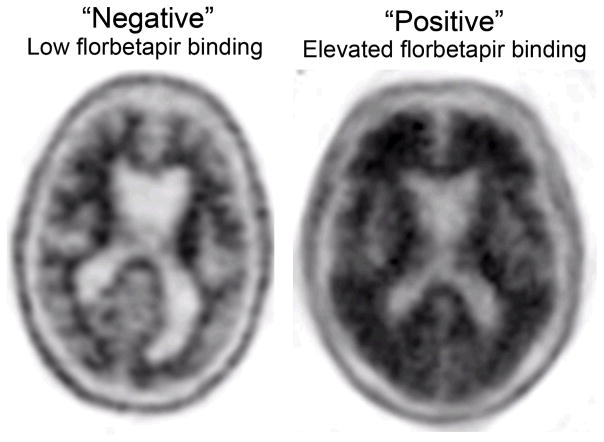
Figure 3.
Assessing amyloid deposition using florbetapir (Amyvid™, http://www.amyvid.com). The axial PET image on the left shows normal preserved gray-white contrast with the cortical radioactivity less than the adjacent white matter (amyloid ‘negative’ scan). The axial PET image on the right demonstrates areas of decreased gray-white contrast with increased cortical radioactivity that is comparable to the radioactivity in the adjacent white matter (amyloid ‘positive’ scan). The florbetapir scan on the right was acquired on an MCI individual who clinically progressed to dementia with high biomarker probability of AD, as supported by this amyloid positive scan and evidence of neuronal injury on structural MRI (see Figure 1).
Role of biomarkers in guiding clinical management
Biomarker testing can help inform near-term prognosis by providing an objective assessment as to whether neurodegeneration is likely to be present. Whereas cognitive testing validates the patient or caregiver complaint that initiated the clinical visit, vMRI provides an orthogonal measure that is less overlapping with the patient complaint thereby guarding against circularity in concluding that the cognitive problem is due to Alzheimer’s. The presence of brain atrophy on vMRI, together with documented memory impairment confirmed by cognitive testing, suggests a prognosis of near-term decline and can prompt a discussion on evaluating the risk/benefit ratio for considering aggressive disease management versus symptomatic care (Figure 2). For patients and family members, these findings can help initiate a dialogue on future planning including determining the need for residential and driving support, involvement of a geriatric case manager, and financial decisions.
Evaluation with amyloid testing can prove useful once a neurodegenerative etiology for cognitive decline has been established, especially in younger patients and in patients presenting with complaints atypical for AD. An amyloid test may be helpful for making a more informative dementia diagnosis (e.g. AD vs. FTD) in these patients, and can help guide the selection of medications for symptomatic management. As with vMRI, amyloid testing may also be of benefit to refine and tailor expectations while providing additional education to patients and caregivers.
The absence of brain atrophy on vMRI confers a better near-term prognosis and can provide cautious, but increased, optimism to physicians, patients and caregivers. While not excluding the possibility of future neurodegeneration, normal brain volumes can guide clinical management by prompting the physician to intensify efforts towards detecting a treatable cause for the patient’s memory impairment (Figure 2). Such physician optimism is not lost on patients and may serve as needed reassurance to those patients with an inappropriately debilitating fear about progressing to dementia. Importantly, the intensified physician effort on behalf of patients whose complaints and cognitive impairments are incongruous with vMRI findings may lead to an improved likelihood of successful treatment and subsequent return of patients to normal cognitive function.
Potential pitfalls with biomarker testing
In addition to valid concerns of added expense (see Table 1b), it is our opinion that biomarker assessment of patients without objective evidence of memory impairment could cause potential harm. For example, given the high frequency of non-specific memory complaints in the general population and the high prevalence of amyloid positivity among the cognitively normal population, there is a significant chance that a patient’s memory complaint is unrelated to intra-cranial Aβ deposition. A finding of elevated amyloid or low hippocampal volume might lead to inappropriate attribution of memory complaints to AD, circumventing a thorough workup for other potentially treatable causes while exacerbating the debilitating worry that initially brought the patient to the clinic. Even in those patients where memory impairment is clinically confirmed, elevated amyloid does not assure that the cause of the impairment is AD. Amyloid positivity, in patients with objective memory decline, might lead to an overly simplistic attribution of memory complaints to AD and incomplete evaluations for modifiable causes of cognitive impairment. Finally, a negative amyloid test is not necessarily a result to be celebrated since other neurodegenerative disorders should remain under consideration.
Table 1.
(a)Clinical features and (b) disease progression markers in amnestic MCI individuals.
| Table 1a
| |
|---|---|
| Clinical Characteristics | |
| Memory Impairment | Episodic memory dysfunction |
| Non-memory Cognitive Impairment | Executive dysfunction, apraxia, aphasia, and/or visuospatial dysfunction maybe present in amnestic MCI multi-domain |
| Functional Impairment | No change in ability to perform activities of daily living |
| Behavioral Impairment | Depression and anxiety maybe present |
| Annual Rate of Progression to Dementia | Variable (range 3%–15%) 1 |
| Table 1b | |||
|---|---|---|---|
| Markers of Disease Progression | Characteristics | Procedure(s)# | Approximate Cost (in US dollars)% |
| Structural Neuroimaging with vMRI | Medial temporal lobe and/or neocortical atrophy, white matter abnormalities may also be present |
|
|
| FDG-PET | Temporoparietal hypometabolism | Brain Imaging (PET) Metabolic Evaluation CPT 78608 | 1266.40 (1041.99 T+150 L+74.41 P) |
| Amyloid Imaging | Increased uptake in frontal, parietal, and/or temporal regions | PET Imaging Limited Area CPT 78811 | 2721.83 (1041.99T+1600L+79.84 P) |
| CSF Amyloid CSF Tau (total tau) |
Decreased Increased |
|
|
| APOE ε4 carrier status | Dose-dependent effect (risk of AD: ε4/ε4 > ε3/ε4 > ε3/ε3 > ε3/ε2 > ε2/ε2) |
|
|
Determined using data from the Centers for Medicare and Medicaid Services (www.cms.gov) For informational purposes only. Selected CPT code may vary.
Determined, when possible, using National Payment Amount data from the Centers for Medicare and Medicaid Services (www.cms.gov) For informational purposes only. Payment amount varies by location.
Using NeuroQuant® (http://www.cortechs.net/products/neuroquant.php)
Using the ADmark® Phospho-Tau/Total-Tau/Ab42 CSF Analysis & Interpretation (Symptomatic) test (http://www.athenadiagnostics.com/content/test-catalog/find-test/service-detail/q/id/311)
Using the ADmark® ApoE Genotype Analysis & Interpretation (Symptomatic) (http://www.athenadiagnostics.com/content/test-catalog/find-test/service-detail/q/id/35)
Approximate Technical Charge
Approximate Professional Charge
Approximate Facility Price
Approximate Ligand Price
Future Directions – Preclinical AD
Currently, there are no effective treatments that delay the onset or halt the progression from MCI to AD. There is increasing recognition that early intervention before the onset of neurodegeneration or clinical symptoms may represent the most effective treatment against AD 19 and a number of secondary prevention trials in preclinical older individuals are currently underway. We believe that a screening strategy for assessing dementia risk in cognitively normal adults could be useful if a meaningful therapy with minimal side effects becomes clinically available. Biomarker testing in asymptomatic patients is inherently controversial and we therefore note that this evaluation strategy, though not currently applicable, may become relevant when/if meaningful preventative interventions are available.
Genetic, biochemical, and imaging evidence indicates that fibrillar Aβ pathology begins at least 15 years before the onset of clinical symptoms. 48 Increasing levels of Aβ oligomers that progressively lead to plaque deposition are likely present at an even earlier age. 49 These observations suggest that screening for the presence of amyloid should start in cognitively normal older adults (greater than 60 years of age), similar to the current screening strategies for hypercholesterolemia or common cancers such as breast, colon or prostate carcinoma. Though CSF concentrations of Aβ may become aberrant before amyloid imaging, 48 additional factors such as the need for assessing therapeutic response over time, clinical availability, and patient comfort should also be considered when determining whether to use fluid or imaging markers for amyloid status screening.
In cognitively normal older adults, a negative amyloid test indicates a significantly lower risk of developing AD. Since increased amyloid tracer uptake can also be seen with other conditions, such as cerebral amyloid angiopathy, 32 a positive amyloid test could be further evaluated using cognitive testing and possibly, vMRI. Positive amyloid status along with the presence of progressive medial temporal lobe atrophy would suggest that the patient has entered the neurodegenerative phase of the disease process, which would change the risk/benefit calculation in considering more aggressive, less benign medications that may become available. Although neuropathology remains the only way to definitively diagnose AD, available fluid and imaging markers supplement the physician toolbox for managing and educating patients and families worried about AD. As disease-modifying therapies are developed, this physician toolbox will likely evolve to further address the need for improved predictive prognosis and disease management in preclinical AD.
CLINICAL PERSPECTIVE.
Late onset Alzheimer’s disease (AD) is the most common form of dementia with an estimated prevalence of 30 million people worldwide, a number that is expected to quadruple in 40 years. With increasing awareness that symptoms develop over many years, there is a growing need to identify non-demented older individuals at risk for AD. Mild cognitive impairment (MCI) represents a transitional state between normal aging and dementia. Clinical features of amnestic MCI are presented in Table 1a and reviewed in references 1 and 2. In this piece, we focus on recent advances in biomarker development for the predictive prognosis of MCI and suggest that a neuroimaging-based evaluation strategy can help guide clinical management decisions in older individuals with memory impairment.
Acknowledgments
Funding: National Institutes of Health grant T32 EB005970
We thank Drs. Linda McEvoy, Dominic Holland, Gil Rabinovici, John Morris, Brad Hyman, Marilyn Albert, William Dillon, Bruce Fischl, Reisa Sperling, Keith Johnson, Clifford Jack, William Bradley, John Hesselink, Ole Andreassen and Anders Dale for helpful input on an earlier draft of this manuscript. This work was supported by a grant from the National Institutes of Health (T32 EB005970).
Footnotes
Disclosures
Drs. Desikan, Rafii, and Hess report no conflicts of interest. Dr. Brewer has a financial interest in companies developing Amyvid and NeuroQuant, two Alzheimer’s disease biomarkers that are reviewed in this manuscript. Specifically, Dr. Brewer holds stock options in CorTechs Labs, Inc (NeuroQuant) and serves on the advisory board and receives financial support from the Eli Lilly Biomarker Unit (Amyvid). Dr. Brewer also receives research support from General Electric and Janssen Alzheimer Immunotherapy.
References
- 1.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: ten years later. Arch Neurol. 2009 Dec;66:1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC. Clinical Practice. Mild Cognitive Impairment N Eng J Med. 2011 Jun 9;364:2227–34. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr. 1907;64:146–148. [Google Scholar]
- 4.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011 Apr 6;3(77):77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):4012–7. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008 Jul 31;59(2):214–25. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;64:632–44. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012 Jul 11; doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 9.Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988 Nov;38(11):1682–7. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 10.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992 Mar;42(3 Pt 1):631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 11.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 14.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM Alzheimer’s Disease Neuroimaging Initiative. Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011 Oct;70(4):657–61. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM for the Alzheimer’s Disease Neuroimaging Initiative. Amyloid-β-Associated Clinical Decline Occurs Only in the Presence of Elevated P-tau. Arch Neurol. 2012 Apr 23; doi: 10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011 Aug;68(8):1062–4. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001 Oct;17(2):101–18. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 19.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56:1143–53. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 21.Vemuri P, Jack CR., Jr Role of structural MRI in Alzheimer’s disease. Alzheimers Res Ther. 2010 Aug 31;2(4):23. doi: 10.1186/alzrt47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR., Jr MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008 Sep 2;71(10):743–9. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004 Jan;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 25.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 Jul 1;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.McEvoy LK, Brewer JB. Quantitative structural MRI for early detection of Alzheimer’s disease. Expert Rev Neurother. 2010 Nov;10(11):1675–88. doi: 10.1586/ern.10.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland D, McEvoy LK, Dale AM. Unbiased comparison of sample size estimates from longitudinal structural measures in ADNI. Hum Brain Mapp. 2011 Nov;33(11):2586–602. doi: 10.1002/hbm.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer JB, Magda S, Airriess C, Smith ME. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol. 2009 Mar;30:578–80. doi: 10.3174/ajnr.A1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacskai BJ, Hickey GA, Skoch J, Kajdasz ST, Wang Y, Huang GF, Mathis CA, Klunk WE, Hyman BT. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12462–7. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Shao L, Hamilton RL, Ikonomovic MD, DeKosky ST, Mathis CA. The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci. 2003 Mar 15;23(6):2086–92. doi: 10.1523/JNEUROSCI.23-06-02086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, Irizarry MC, Klunk WE, Mathis CA, Dekosky ST, Greenberg SM, Hyman BT, Growdon JH. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007 Mar;64(3):431–4. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 33.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008 Jun;131(Pt 6):1630–45. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21:117–28. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012 Apr;2(4):a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtzman DM. CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol Aging. 2011 Dec;32( Suppl 1):S4–9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laino C. News from the American Neurological Association Annual Meeting: Anti-Amyloid-Beta Drug Modestly Slows Cognitive Decline in Mild to Moderate AD. Neurology Today. 2012 Nov 1;12(21):34–38. [Google Scholar]
- 38.Jack CR, Jr, Barkhof F, Bernstein MA, Cantillon M, Cole PE, Decarli C, Dubois B, Duchesne S, Fox NC, Frisoni GB, Hampel H, Hill DL, Johnson K, Mangin JF, Scheltens P, Schwarz AJ, Sperling R, Suhy J, Thompson PM, Weiner M, Foster NL. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement. 2011 Jul;7(4):474–485.e4. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR., Jr Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012 May;263(2):344–61. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010 Nov;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 41.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Highlights of prescribing information: Amyvid (florbetapir F18 injection) Silver Spring, MD: Food and Drug Administration; ( http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202008s000lbl.pdf) [Google Scholar]
- 44.Mucke L, Selkoe DJ. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009 Dec;66(12):1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schott JM, Bartlett JW, Fox NC, Barnes J Alzheimer’s Disease Neuroimaging Initiative Investigators. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann Neurol. 2010 Dec;68(6):825–34. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 47.Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, Fagan AM, Head D, Mintun MA, Ladenson JH, Lee JM, Morris JC, Holtzman DM. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011 Aug;70(2):274–85. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC the Dominantly Inherited Alzheimer Network. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 Jul 11; doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17:1060–5. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 50.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF American Heart Association; American Stroke Association Stroke Council; Clinical Cardiology Council; Cardiovascular Radiology and Intervention Council; Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007 May;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 51.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009 Mar 24;72:1048–55. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costafreda SG, Dinov ID, Tu Z, Shi Y, Liu CY, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Wahlund LO, Spenger C, Toga AW, Lovestone S, Simmons A. Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage. 2011 May 1;56:212–9. doi: 10.1016/j.neuroimage.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.den Heijer T, van der Lijn F, Koudstaal PJ, Hofman A, van der Lugt A, Krestin GP, Niessen WJ, Breteler MM. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010 Apr;133:1163–72. doi: 10.1093/brain/awq048. [DOI] [PubMed] [Google Scholar]
- 54.Desikan RS, Cabral HJ, Fischl B, Guttmann CR, Blacker D, Hyman BT, Albert MS, Killiany RJ. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of Alzheimer disease. AJNR Am J Neuroradiol. 2009 Mar;30:532–8. doi: 10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK Alzheimer’s Disease Neuroimaging Initiative. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011 Oct 25;77:1619–28. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS Alzheimer’s Disease Neuroimaging Initiative. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010 Nov;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovacevic S, Rafii MS, Brewer JB Alzheimer’s Disease Neuroimaging Initiative. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2009 Apr-Jun;23:139–45. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluimer JD, van der Flier WM, Karas GB, van Schijndel R, Barnes J, Boyes RG, Cover KS, Olabarriaga SD, Fox NC, Scheltens P, Vrenken H, Barkhof F. Accelerating regional atrophy rates in the progression from normal aging to Alzheimer’s disease. Eur Radiol. 2009 Dec;19:2826–33. doi: 10.1007/s00330-009-1512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR, Jr Alzheimer’s Disease Neuroimaging Initiative. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009 Jul 28;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westman E, Cavallin L, Muehlboeck JS, Zhang Y, Mecocci P, Vellas B, Tsolaki M, Koszewska I, Soininen H, Spenger C, Lovestone S, Simmons A, Wahlund LO AddNeuroMed consortium. Sensitivity and specificity of medial temporal lobe visual ratings and multivariate regional MRI classification in Alzheimer’s disease. PLoS One. 2011;6:e22506. doi: 10.1371/journal.pone.0022506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011 Apr 19;76:1395–402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickerson BC, Wolk DA Alzheimer’s Disease Neuroimaging Initiative. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012 Jan 10;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEvoy LK, Brewer JB. Biomarkers for the clinical evaluation of the cognitively impaired elderly: amyloid is not enough. Imaging Med. 2012 Jun;4:343–357. doi: 10.2217/iim.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]