Abstract
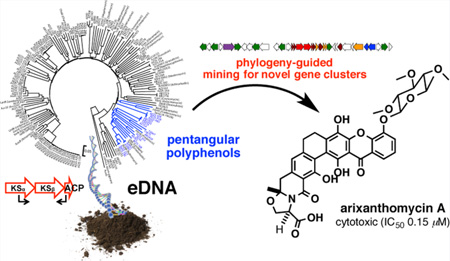
Soil microbiomes are a rich source of uncharacterized natural product biosynthetic gene clusters. Here we use short conserved biosynthetic gene sequences (natural product sequence tags) amplified from soil microbiomes as phylogenetic markers to correlate genotype to chemotype and target the discovery of novel bioactive pentangular polyphenols from the environment. The heterologous expression of an environmental DNA-derived gene cluster (the ARX cluster), whose ketosynthase beta (KSβ) sequence tag was phylogenetically distinct from any known KSβ sequence, led to the discovery of the arixanthomycins. Arixanthomycin A (1) exhibits potent antiproliferative activity against human cancer cell lines.
Microbially based natural products discovery programs have traditionally relied on the random screening of a pool of novel biodiversity (i.e., new microbial species) to secure a source of structurally novel small molecules.1 As most bacteria in the environment have not yet been cultured, libraries of DNA extracted directly from environmental samples (environmental DNA, eDNA) represent large reservoirs of unexplored natural product biosynthetic gene clusters that could serve as new sources of additional bioactive small molecules.2,3 Advances in modern sequencing technologies, bioinformatics analysis tools, and environmental DNA cloning methods should allow for a movement in natural products discovery away from a random microbe screening approach to a more systematic sequence-based screening approach that will allow for the functional examination of gene clusters that remain hidden in the environment.4,5 In its simplest version, we envision that this sequence-based approach will use highly conserved biosynthetic genes as phylogenetic markers to identify novel gene clusters that can serve as starting points for the discovery of novel bioactive natural products. Here, we report the use of a single gene phylogeny-based mining approach to guide the discovery of arixanthomycins A–C (1–3, Chart 1), new pentangular polyphenols that exhibit potent in vitro antiproliferative activity.
Chart 1.
Pentangular polyphenols are a subfamily of aromatic polyketides (PK) that use C24 to C30 polyacetate precursors.6 Among characterized biosynthetic pathways, these are the longest polyketide chains to be generated by minimal type II polyketide synthases (min-PKSs).7 Known pentangular poly-phenols exhibit a wide range of biological activities, including antibacterial, antiproliferative, and antifungal activities.8,9 In comparison to the structural diversity reported for many other aromatic PK subfamilies, pentangular polyphenols appear to be underrepresented among known natural products. Their larger size should theoretically afford the ability to generate more diverse folding patterns, skeletal rearrangements, and tailoring modifications than is seen in the biosynthesis of the smaller aromatic PKs. We reasoned that the relative dearth of known pentangular structures, yet high structural potential of this biosynthetic subgroup, would make pentangular polyphenols a rewarding target for phylogeny-guided natural product discovery approaches.
Our search for novel pentangular polyphenols began by PCR amplifying and sequencing ketosynthase beta (KSβ) genes from archived soil eDNA libraries. The KSβ gene encodes a subunit of the min-PKS that is responsible for producing the nonreduced polyketide, which is used as an intermediate in aromatic polyketide biosynthesis. KSβ genes have been shown to be robust phylogenetic markers for structural differences in the natural products encoded by type II PKS gene clusters.5,10
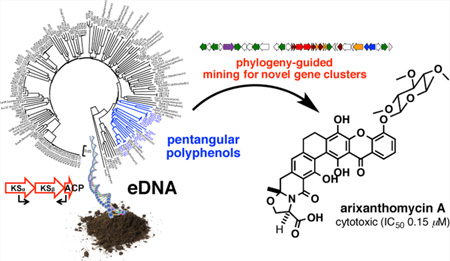
When eDNA-derived KSβ amplicons were mapped onto a phylogenetic tree of functionally characterized KSβ genes, a number of amplicons were found to fall into the well-defined pentangular polyphenol clade, suggesting the presence of pentangular polyphenol biosynthetic gene clusters in our archived eDNA libraries (Figure 1). A close inspection of pentangular polyphenol KSβ gene phylogeny indicates that its divergence correlates strongly with the detailed differences in the functional output of their respective gene clusters (Figure 1).11–18 In other words, this single gene can predict not only the general class of a type II PKS gene cluster (e.g., anthracycline, tetracycline, angucycline, pentangular polyphenol etc.) but also many of the tailoring details encoded within a type II PKS gene cluster.
Figure 1.
Maximum likelihood subtree (1,000 bootstrap replicates) of KSβ genes that clade with known pentangular polyphenol KSβ gene sequences. Based on a comparison of chemical structures and gene cluster contents, biosynthetic relationships in the KSβ tree have been grouped into color-coded boxes. Natural pentangular polyphenol structural diversification appears to be dictated by four major driving forces: (i) start unit selection, (ii) initial cyclization pattern, (iii) oxidative rearrangement of the core skeleton, and (iv) functionalization of the terminal carboxylic acid. On the basis of this analysis, we hypothesized that phylogenetic comparison of eDNA-derived KSβ sequence tags to KSβ genes from functionally characterized gene clusters should be sufficient to enable detailed structural predictions (chemotype) of metabolites encoded by eDNA-derived gene clusters (genotype). The eDNA-derived KSβ sequence AZ33 is shown in red.
The strong correlation between final structural differences and KSβ gene sequence phylogeny enabled us to prioritize eDNA-derived pentangular polyphenol gene clusters for heterologous expression experiments based solely on our initial PCR screening. The eDNA-derived KSβ sequences of initial interest to us were those that fell into the subclade characterized by the presence of a Baeyer–Villiger oxidase. This enzyme is often used for carbon skeleton remodeling and thus is responsible for introducing significant structural and biological activity diversity into the pentangular polyphenol family (e.g., rubromycin, griseorhodin, lysolipin, and xantholipin).11,12,14,18 Among the four eDNA-derived KSβ sequences that fell into this subclade, AZ25_235 and AZ957 showed high sequence identity to KSβ sequences from rubromycin biosynthesis (96% and 91%, respectively), suggesting that the eDNA gene clusters from which they arise likely encode either rubromycin itself or simple derivatives of this structure.12 In contrast, the AZ33 KSβ sequence appears to define a new branch within the Baeyer–Villiger oxidase containing subclade, suggesting that AZ33 was likely associated with the biosynthesis of a novel pentangular polyphenol subclass.
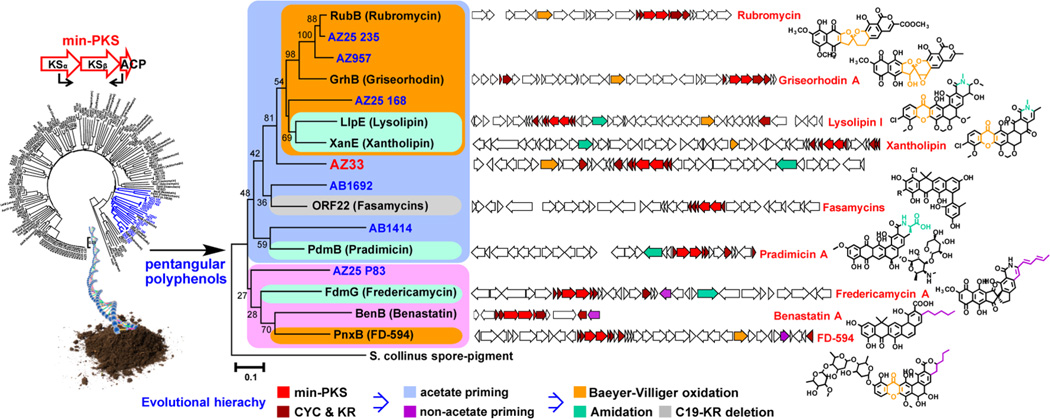
The AZ33 gene cluster was recovered on three overlapping cosmid clones (AZ1076, AZ33, and AZ378) from the archived Arizona desert eDNA library from which the AZ33 KSβ sequence tag was amplified (Figure 2). To permit heterologous expression studies, these three overlapping clones were assembled into one bacterial artificial chromosome (BAC-AZ1076/33/378) by transformation-associated recombination (TAR) in yeast (Figure 2).19,20 The correct assembly of the AZ33 cluster was confirmed by de novo sequencing of BAC-AZ1076/33/378. This BAC construct was then transferred into Streptomyces albus via intergenic conjugation and integrated into the genome by ΦC 31 integrase to generate the strain S. albus BAC-AZ1076/33/378. For natural product isolation studies, this strain was grown at 30 °C in R5A media for 7 days with shaking, and the resulting cultures were extracted with ethyl acetate. S. albus BAC-AZ1076/33/378 culture broth extract showed a potent antiproliferative activity against human carcinoma cell lines (Table 1), and LC–MS analysis of the extract showed the presence of three gene cluster-specific molecules. Each molecule was predicted by HRMS to have a molecular formula that was distinct from any previously isolated pentangular polyphenol natural product (Figure 2). On the basis of the potent crude extract bioactivity, the bioinformatics, and chemical novelty, we chose to carryout a detailed structure analysis of the clone specific metabolites produced by S. albus BAC-AZ1076/33/378.
Figure 2.
TAR reassembly of three overlapping eDNA clones carrying the ARX pentangular polyphenol pathway and heterologous expression of BAC-AZ1076/33/378.
Table 1.
Anticancer, Antifungal, and Antibacterial Activities of 1–3
| crudea | 1 | 2 | 3 | ||
|---|---|---|---|---|---|
| anticancerb | HCT-116 | 1.1 | 0.15 | 5.14 | 25.42 |
| WiDr | 2.5 | 0.83 | >50 | >50 | |
| MDA-MB-231 | 1.4 | 0.25 | 9.36 | >50 | |
| antifungalc | S. cerevisiae | >50 | >50 | >50 | |
| antibacterialc | MRSA USA300 | 1.6 | 25 | >50 | |
| B. subtilis RM125 | 3.1 | 12.5 | >50 | ||
| VRE EF16 | 50 | >50 | >50 | ||
| E. coli DRC39 | >50 | >50 | >50 |
IC50 in µg/mL.
IC50 in µM.
MIC in µg/mL.
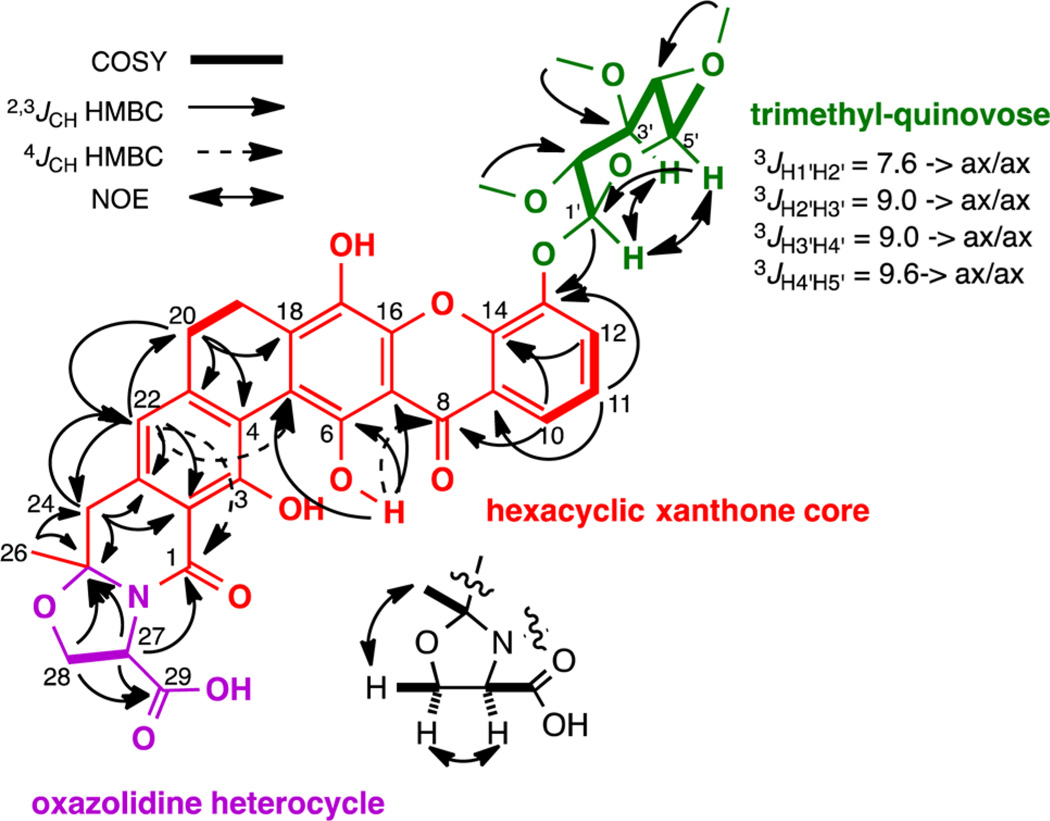
The three clone specific metabolites were purified from large-scale cultures (1 L) broth extracts using C18 reversed-phase HPLC to yield compounds 1 (1.9 mg L−1), 2 (4.6 mg L−1), and 3 (4.0 mg L−1). Compounds 1–3 were named arixanthomycins A, B, and C, respectively. The planar structures and relative configurations of arixanthomycins A–C (1–3) were determined by a combination of HRESIMS and 1D and 2D NMR data including 1H, 13C, COSY, HMQC, HMBC, and NOESY (Figure 3; Supporting Information includes a detailed structure elucidation discussion).
Figure 3.
2D NMR data used to define the structure of arixanthomycin A (1). The configurations shown for the oxazolidine ring and sugar moieties are relative.
The arixanthomycins feature a hexacyclic xanthone core (Figure 3, red) that is predicted to arise from a Baeyer–Villiger oxidative rearrangement of a pentangular polyphenol precursor as would be expected from the position of the AZ33 KSβ gene sequence in the KSβ phylogenetic tree. However, as we anticipated from the position of the AZ33 KSβ gene sequence on a new branch in the phylogenetic tree, the arixanthomycins differ from known members of the xanthone family pentangular polyphenols by a number of key features. These differences include the presence of the carboxylated oxazolidine ring (Figure 4, G ring), the oxidation state of the D ring, the oxidation state of the F ring, and the glycosylation at C-13 (Figure 4, green). The oxazolidine ring likely arises from the coupling of a serine to the terminal polyketide carboxylic acid (Figure 4). The kigamicins and cervinomycins are the only members of the pentangular polyphenol family that possess an oxazolidine G ring.21–23 In these known metabolites, the oxazolidine rings would appear to arise from the incorporation of an ethanolamine and therefore the carboxylate is not present. While sugars are seen on some pentangular polyphenols,23–25 no previous reports of a C-13 glycosylation appear in the literature.
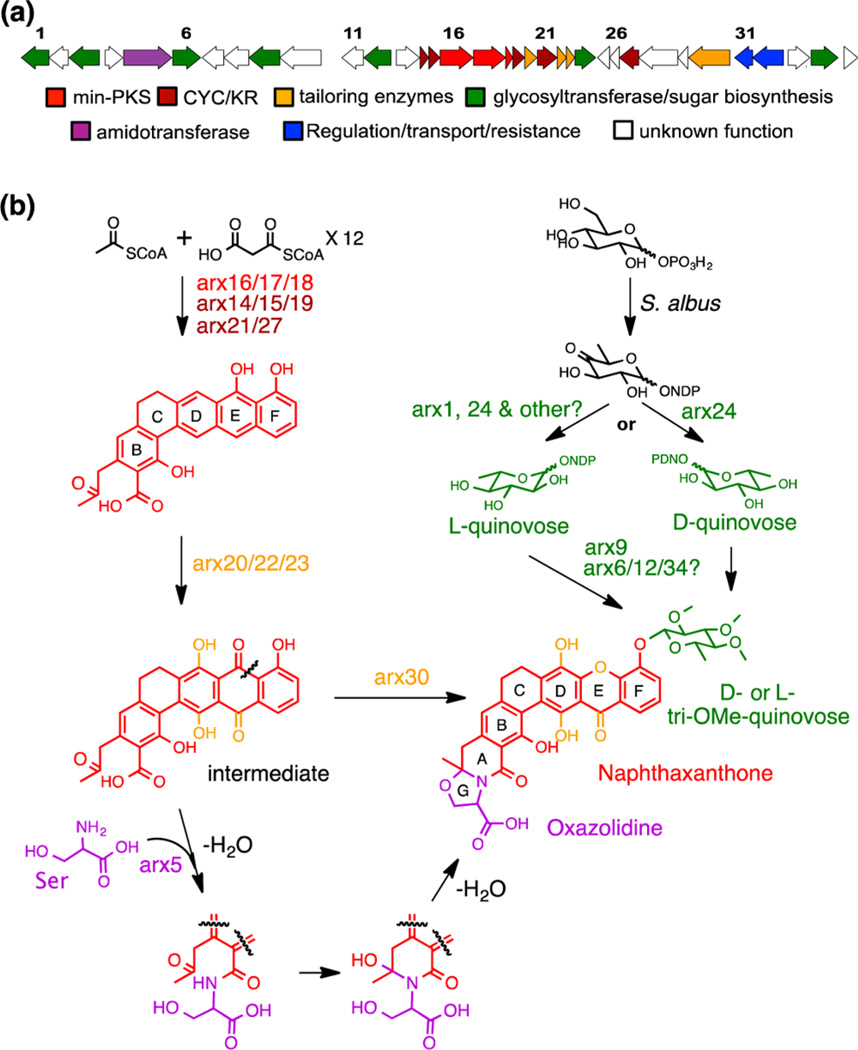
Figure 4.
(a) ARX (arixanthomycin) gene cluster (GenBank accession no. KF931341). (b) Proposed biosynthetic scheme for arixanthomycin A (1) based on gene function prediction defined by sequence homology and domain analysis.
Arixanthomycins A–C (1–3) were assayed for antiproliferative, antibacterial, and antifungal activities. The arixanthomycins were found to be most active in antiproliferative assays (Table 1). Arixanthomycin A (1) shows a particularly potent antiproliferative activity against colon (HCT-116 and WiDr) and breast (MDA-MB-231) cancer cell lines (Table 1). Arixanthomycins B (2) and C (3) are significantly less active in these assays, suggesting that the sugar moiety is important for the antiproliferative activity of 1. This is rather surprising, as most xanthone-containing pentangular polyphenols show potent activity in the absence of a sugar moiety.25–29 Whether this is an indication that arixanthomycin A (1) has a distinct mode of action from other pentangular polyphenols or that the sugar is needed to improve the bioavailability of 1 in light of the new carboxylate remains to be determined.
The biosynthesis of the arixanthomycins can be rationalized on the basis of the predicted function of the genes found within BAC-AZ1076/33/378 (Figure 4). The predicted ARX gene cluster spans 33 kb and contains 35 genes predicted to be involved in the biosynthesis, regulation, and resistance of the arixanthomycins. The structure of arixanthomycin A (1) can be divided into three parts, the naphthaxanthone core, the oxazolidine ring, and the quinovose sugar moiety. Key ARX enzymes that are predicted to be involved in the biosynthesis of each of these substructures are shown in Figure 4. The hexacyclic naphthaxanthone core is predicted to arise from a pradimicin-type anthraquinone intermediate by a Baeyer–Villiger oxidation catalyzed by Arx30 (62% identity to llpOVIII and 61% identity to xanO4).11,14 Stereospecific formation of the oxazolidine (G ring) is likely to occur spontaneously upon incorporation of serine into the terminal carboxylate by the predicted amidotransferase Arx5. The addition of the trimethylated quinovose sugar moiety is predicted to occur through the action of the glycosyltransferase Arx9. A detailed biosynthetic proposal appears in the Supporting Information (Figure S3).
This work demonstrates the power of using single gene sequence tags to rapidly and economically bridge the gap between genotype and chemotype in complex microbiomes. This methodology is easy to scale using high throughput sequencing technology and archived eDNA libraries. Sequence-based eDNA mining approaches like those used here should allow for the movement away from the traditional new species-based natural product discovery paradigm to a new bioinformatics-driven, clade-based, natural products discovery paradigm. Using this general approach, it should be possible to systematically access untapped chemical diversity encoded within diverse natural microbiomes.
METHODS
Clone Recovery and Bioinformatics Analysis
eDNA clone AZ33 was recovered from the AZ eDNA library by serial dilution of the 5,000-member subpool from which the AZ33 amplicon was originally amplified. To do this, an overnight culture of the AZ33 subpool was inoculated into 96-well microtiter plates at a dilution of 10−6. After 18 h of shaking at 37 °C, the diluted cultures were screened by whole-cell PCR using the KSβ screening primers and touchdown PCR protocol. PCR positive wells were plated onto solid media (LB agar) to yield distinct colonies that were screened individually in a second round of whole-cell PCR. Overlapping clones for AZ33 were subsequently identified in the AZ library using clone-specific primers designed to recognize the sequence at each end of AZ33 (AZ33F_FW: CAGGAACTCGAGTCCATCTC, AZ33F_RV: ACTTCACCAGTACCTCGAC, AZ33B_FW: CACATGAAGCTGATGACTC, and AZ33B_RV: GCCATTCGATCTCGATAC). The PCR protocol used was as follows: denaturation (95 °C, 5 min), 36 standard cycles (95 °C, 30 s; 55 °C, 30 s; 72 °C, 40s), and a final extension step (72 °C, 7 min). Hits were identified in subpools AZ1076 and AZ378. Overlapping eDNA cosmids (designated AZ1076 and AZ378) were recovered from these pools using the serial dilution method outlined above. All three cosmids were sequenced by 454-pyrosequencing. ORFs (open reading frames) were predicted using MetaGeneMark,30 and predicted ORFs were annotated by Blast search and Pfam domain analysis.
TAR Assembly of Overlapping eDNA Clones
The three overlapping eDNA cosmid clones that are predicted to contain the AZ33 gene cluster (AZ1076, AZ33 and AZ378) were assembled into a single bacterial artificial chromosome (BAC) clone using transformation-associated recombination (TAR) in yeast and the pTARa BAC shuttle vector.19,20 The pathway-specific TAR capture vector was constructed using InFusion cloning methodology (Clontech). Five hundred bases upstream and downstream homology arms were amplified from the AZ1076 and AZ378 cosmids, respectively, using the following primers:
AZ1076UPS_FW (5′-CTATCGATCTCGAGGACGACCTG-ATGAGCAGTA-3′),
AZ1076UPS_RV (5′-CGTGCCACTGTTAACTCAACTCC-ATCCTCACCT-3′),
AZ378DWS_FW (5′-GTTAACAGTGGCACGTTGACGTTCTT-3′),
AZ378DWS_RV (5′-CCCTGCAGGAGCTCGTCTCTTTCTC-GTCCACTGGT-3′)
Primers AZ1076UPS_FW and AZ1076DWS_RV include 15 bp sequences (underlined) that overlap with BmtI/SphI linearized pTARa capture vector. Primer AZ1076UPS_RV was designed to contain a 15 bp overlap (underlined) with the AZ378DWS_FW primer and an HpaI site (bold), which was added to facilitate the subsequent linearization of the pathway-specific pTARa capture vector. Gel-purified amplicons (Qiagen) and BmtI/SphI linearized pTARa were combined in a standard InFusion cloning reaction (Clontech) to yield the pathway-specific capture vector.
For TAR assembly, 200 ng of DraI-digested AZ1076, AZ33, and AZ378 were mixed with 100 ng of the HpaI-cut pathway-specific capture vector and then transformed into 200 µL of Saccharomyces cerevisiae CRY1-2 spheroplasts prepared according to published protocols.19,20 Transformed spheroplasts were mixed with synthetic complete (SC) top agar (1 M sorbitol, 1.92 g/L synthetic complete uracil dropout supplement, 6.7 g/L yeast nitrogen base, 2% glucose, and 2.5% agar) and overlaid onto SC uracil dropout plates. Plates were incubated at 30 °C for 72 h. DNA was prepared from 12 yeast colonies using a zymolyase lysis protocol (ZYMO RESEARCH) and screened by PCR with primers designed to recognize sequences from AZ1076, AZ33, and AZ378. A BAC clone that was “PCR positive” with all the primer sets was electroporated into E. coli EPI300. DNA isolated from the resulting E. coli was PGM (Ion Torrent Personal Genome Machine) sequenced to confirm the correct reassembly of the three overlapping cosmids into a 80 kb insert BAC clone (BAC-AZ1076/33/378).
Heterologous Expression
BAC-AZ1076/33/378 was transformed into E. coli S17.1 and conjugated into S. albus to generate S. albus BAC-AZ1076/33/378 (apramycin selection). This strain was cultured in R5A31 liquid medium for 7 days (200 rpm at 30 °C) and then extracted with ethyl acetate (1/3 volume to the culture broth). The resulting ethyl acetate extract was compared by HPLC to the extract from a similarly grown culture of S. albus transformed with a pTARa empty vector control (HPLC conditions: 30 min gradient from 5% to 100% aqueous acetonitrile containing 0.1% trifluoroacetic acid, C18, 4.6 mm × 150 mm, 1 mL/min).
Extraction and Isolation
Compounds 1–3 were isolated from cultures of S. albus BAC-AZ1076/33/378 grown (200 rpm at 30 °C) in 125 mL baffled flasks containing 50 mL of R5A media. Seven-day-old cultures were extracted with ethyl acetate (1/3, v/v to the culture broth), and the ethyl acetate was dried in vacuo. Arixanthomycins A (1), B (2), and C (3) were isolated from the resulting ethyl acetate extract using two rounds of reversed-phase HPLC (C18 column, 10 mm × 250 mm, 3.5 mL/min). The first round of HPLC (isocratic, 55% acetonitrile containing 0.1% trifluoroacetic acid) yielded three crude compounds 1 (17.9 min), 2 (9.8 min), and 3 (6.6 min), which were repurified by a second round of HPLC using 80%, 72%, and 70% aqueous methanol with 0.1% trifluoroacetic acid to yield arixanthoymcins A (1, 1.6 mg L−1), B (2, 4.6 mg L−1), and C (3, 4.1 mg L−1), respectively.
Arixanthoymcin A (1)
Yellowish powder; [α]25D −197; UV (MeOH) λmax (log ε) 287 (4.24), 327 (shoulder; 4.13), 346 (shoulder; 3.98), 403 (3.57) nm; IR (neat) νmax 3375, 2981, 2945, 2912, 2835, 1680, 1641, 1631, 1606, 1548, 1529, 1492, 1462, 1431 cm−1; 1H and 13C NMR, COSY, and HMBC data, see Supplementary Table 1; HRESIMS m/z 720.2300 [M + H]+ (calcd for C37H38 NO14, 720.2292).
Arixanthomycin B (2)
Greenish powder; [α]25D −127; UV (MeOH) λmax (log ε) 285 (4.42), 331 (shoulder; 4.12), 399 (3.47) nm; IR (neat) νmax 3110, 2980, 2891, 2846, 1681, 1614, 1586 1488, 1467, 1437 cm−1; 1H and 13C NMR data, see Supplementary Table 2; HRESIMS m/z 546.1379 [M + H]+ (calcd for C29H24NO10, 546.1400).
Arixanthoymcin C (3)
Yellowish powder; [α]25D −351; UV (MeOH) λmax (log ε) 287 (4.74), 324 (shoulder; 4.55), 347 (shoulder; 4.40), 399 (3.90) nm; IR (neat) νmax 3429, 2982, 2940, 2893, 2837, 1679, 1640, 1610, 1590, 1492, 1440 cm−1; 1H and 13C NMR data, see Supplementary Table 2; HRESIMS m/z 532.1246 [M + H]+ (calcd for C28H22NO10, 532.1244).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant GM-77516. S.F.B. is an HHMI Early Career Scientist.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Methods for library construction, library screening, and bioassays and discussions for structure determination. NMR data table, gene annotation table and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handelsman J, Rondon MR, Brady SF, Clardy J, Goodman RM. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 1998;5:R245–R249. doi: 10.1016/s1074-5521(98)90108-9. [DOI] [PubMed] [Google Scholar]
- 3.Reddy BV, Kallifidas D, Kim JH, Charlop-Powers Z, Feng Z, Brady SF. Natural product biosynthetic gene diversity in geographically distinct soil microbiomes. Appl. Environ. Microbiol. 2012;78:3744–3752. doi: 10.1128/AEM.00102-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey A. Strategies for discovering drugs from previously unexplored natural products. Drug Discovery Today. 2000;5:294–300. doi: 10.1016/s1359-6446(00)01511-7. [DOI] [PubMed] [Google Scholar]
- 5.Feng Z, Kallifidas D, Brady SF. Functional analysis of environmental DNA-derived type II polyketide synthases reveals structurally diverse secondary metabolites. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12629–12634. doi: 10.1073/pnas.1103921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackner G, Schenk A, Xu Z, Reinhardt K, Yunt ZS, Piel J, Hertweck C. Biosynthesis of pentangular polyphenols: deductions from the benastatin and griseorhodin pathways. J. Am. Chem. Soc. 2007;129:9306–9312. doi: 10.1021/ja0718624. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Li Y, Tang Y. Cyclization of aromatic polyketides from bacteria and fungi. Nat. Prod. Rep. 2010;27:839–868. doi: 10.1039/b911518h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TJ, Giri N. Pradimicins: a novel class of broad-spectrum antifungal compounds. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:93–97. doi: 10.1007/BF01575126. [DOI] [PubMed] [Google Scholar]
- 9.Winter DK, Sloman DL, Porco JA., Jr Polycyclic xanthone natural products: structure, biological activity and chemical synthesis. Nat. Prod. Rep. 2013;30:382–391. doi: 10.1039/c3np20122h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seow KT, Meurer G, Gerlitz M, Wendt-Pienkowski E, Hutchinson CR, Davies J. A study of iterative type II polyketide synthases, using bacterial genes cloned from soil DNA: a means to access and use genes from uncultured microorganisms. J. Bacteriol. 1997;179:7360–7368. doi: 10.1128/jb.179.23.7360-7368.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Wang L, Kong L, Wang T, Chu Y, Deng Z, You D. Unveiling the post-PKS redox tailoring steps in biosynthesis of the type II polyketide antitumor antibiotic xantholipin. Chem. Biol. 2012;19:422–432. doi: 10.1016/j.chembiol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Bruenker P, Sterner O, Bailey JE, Wolfgang M. Molecular analysis of the rubromycin biosynthesis gene cluster isolated from Streptomyces collinus DSM2012 and synthesis of novel recombinant polyketides. Nippon Nogei Kagakkai Taikai Koen Yoshishu. 2002:299. [Google Scholar]
- 13.Kim BC, Lee JM, Ahn JS, Kim BS. Cloning, sequencing, and characterization of the pradimicin biosynthetic gene cluster of Actinomadura hibisca P157-2. J. Microbiol. Biotechnol. 2007;17:830–839. [PubMed] [Google Scholar]
- 14.Lopez P, Hornung A, Welzel K, Unsin C, Wohlleben W, Weber T, Pelzer S. Isolation of the lysolipin gene cluster of Streptomyces tendae Tu 4042. Gene. 2010;461:5–14. doi: 10.1016/j.gene.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B. Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J. Am. Chem. Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- 16.Kudo F, Yonezawa T, Komatsubara A, Mizoue K, Eguchi T. Cloning of the biosynthetic gene cluster for naphthoxanthene antibiotic FD-594 from Streptomyces sp. TA-0256. J. Antibiot. 2011;64:123–132. doi: 10.1038/ja.2010.145. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Schenk A, Hertweck C. Molecular analysis of the benastatin biosynthetic pathway and genetic engineering of altered fatty acid-polyketide hybrids. J. Am. Chem. Soc. 2007;129:6022–6030. doi: 10.1021/ja069045b. [DOI] [PubMed] [Google Scholar]
- 18.Li A, Piel J. A gene cluster from a marine Streptomyces encoding the biosynthesis of the aromatic spiroketal polyketide griseorhodin A. Chem. Biol. 2002;9:1017–1026. doi: 10.1016/s1074-5521(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Feng Z, Bauer JD, Kallifidas D, Calle PY, Brady SF. Cloning large natural product gene clusters from the environment: piecing environmental DNA gene clusters back together with TAR. Biopolymers. 2010;93:833–844. doi: 10.1002/bip.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallifidas D, Brady SF. Reassembly of functionally intact environmental DNA-derived biosynthetic gene clusters. Methods Enzymol. 2012;517:225–239. doi: 10.1016/B978-0-12-404634-4.00011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omura S, Nakagawa A, Kushida K, Lukacs G. Structure of cervinomycin, a novel antianaerobic antibiotic. J. Am. Chem. Soc. 1986;108:6088–6089. doi: 10.1021/ja00279a095. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa A, Omura S, Kushida K, Shimizu H, Lukacs G. Structure of cervinomycin, a novel xantone antibiotic active against anaerobe and mycoplasma. J. Antibiot. 1987;40:301–308. doi: 10.7164/antibiotics.40.301. [DOI] [PubMed] [Google Scholar]
- 23.Kunimoto S, Someno T, Yamazaki Y, Lu J, Esumi H, Naganawa H. Kigamicins, novel antitumor antibiotics. II. Structure determination. J. Antibiot. 2003;56:1012–1017. doi: 10.7164/antibiotics.56.1012. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez JC, Fernandez Puentes JL, Baz JP, Canedo LM. IB-00208, a new cytotoxic polycyclic xanthone produced by a marine-derived Actinomadura. II. Isolation, physicochemical properties and structure determination. J. Antibiot. 2003;56:318–321. doi: 10.7164/antibiotics.56.318. [DOI] [PubMed] [Google Scholar]
- 25.Ratnayake R, Lacey E, Tennant S, Gill JH, Capon RJ. Kibdelones: novel anticancer polyketides from a rare Australian actinomycete. Chemistry. 2007;13:1610–1619. doi: 10.1002/chem.200601236. [DOI] [PubMed] [Google Scholar]
- 26.Ando T, Chen Z, Chu Y, Kawashima A, Terui Y. Xanthone compound. WO2002027010 A1. Patent. 2002
- 27.Pultar T. PhD thesis. Germany: University of Tübingen; 1988. Lysolipin X und I—Fermentation, Isolierung, Biologische Wirkung und Interaktion mit Mg2+ [Google Scholar]
- 28.Carter GT, Nietsche JA, Williams DR, Borders DB. Citreamicins, novel antibiotics from Micromonospora citrea: isolation, characterization, and structure determination. J. Antibiot. 1990;43:504–512. doi: 10.7164/antibiotics.43.504. [DOI] [PubMed] [Google Scholar]
- 29.Liu LL, He LS, Xu Y, Han Z, Li YX, Zhong JL, Guo XR, Zhang XX, Ko KM, Qian PY. Caspase-3-dependent apoptosis of citreamicin epsilon-induced HeLa cells is associated with reactive oxygen species generation. Chem. Res. Toxicol. 2013;26:1055–1063. doi: 10.1021/tx4000304. [DOI] [PubMed] [Google Scholar]
- 30.Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010;38:e132. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez E, Weissbach U, Sanchez Reillo C, Brana AF, Mendez C, Rohr J, Salas JA. Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J. Bacteriol. 1998;180:4929–4937. doi: 10.1128/jb.180.18.4929-4937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.