Fig. 2.
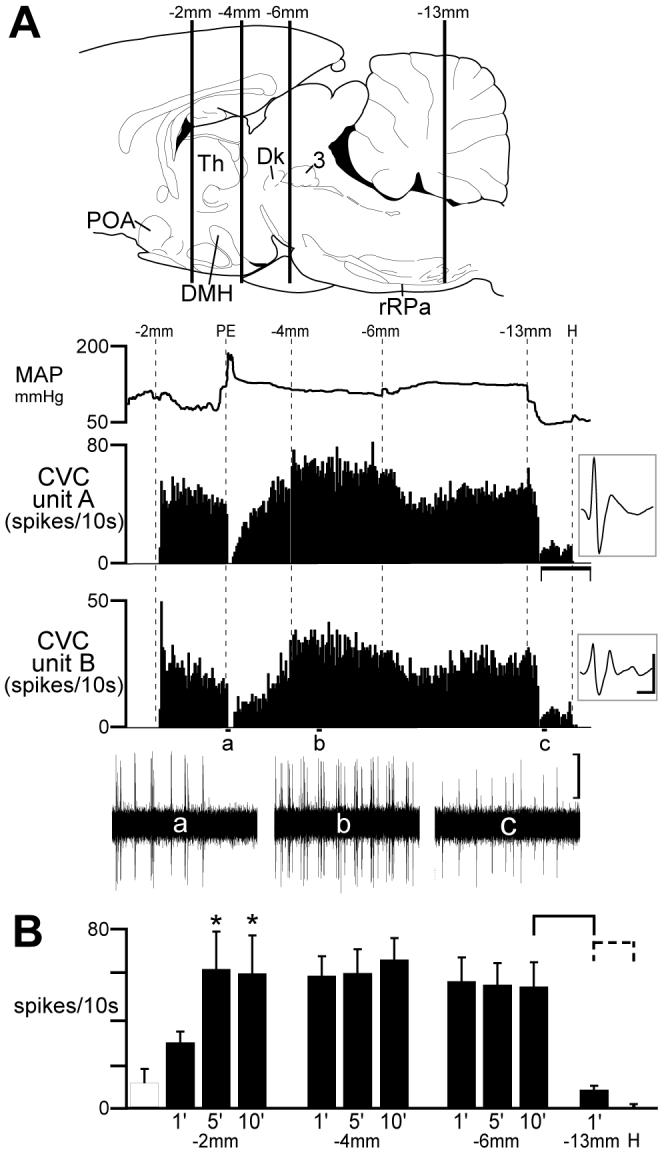
Effect of sequential transections of the neuraxis on the activity of tail cutaneous vasoconstrictor (CVC) postganglionic neurons. A: top image illustrates the approximate positions of serial (rostral to caudal) transections of the neuraxis by vertical lines (labeled as distance caudal to bregma) superimposed on a drawing of the rat brain (44). Traces indicate the time courses of the mean arterial pressure (MAP) and the action potential frequency of two discriminated tail CVC neurons (insets: average of 150 discriminated action potentials, vertical scale: 20 μv, horizontal scale: 2 ms). Phenylephrine (PE)-induced increase in MAP (+90 mmHg from 94 mmHg) elicited a baroreceptor-mediated inhibition of the discharge of the CVC neurons (trace a). Horizontal scale: 10 min. Oscillographic traces of unit action potentials (a-c) are 1 min duration, vertical scale: 20 μv. POA: preoptic area; DMH: dorsomedial hypothalamus; rRPa: rostral raphe pallidus; Th: thalamus; Dk: nucleus of Darkschewitsch; 3: occulomotor nucleus. B: Mean ± SE CVC neuronal discharge frequency following serial transections of the neuraxis. Open hatched bar indicates pre-transection firing frequency. * indicates a significant (P < 0.05) increase above pre-transection level resulting from cut at bregma -2 mm (caudal to the POA). After transections at bregma -4 or -6 mm, mean CVC neuronal activity was not different from that after transection at bregma -2 mm. Transection at bregma -13 mm (caudal to the rRPa) significantly reduced CVC neuronal activity (solid bracket: P < 0.01). Hexamethonium eliminated CVC neuronal activity (dashed bracket: P < 0.05).
