Table 2.
Scope of Pd-Catalyzed Carboaminationa
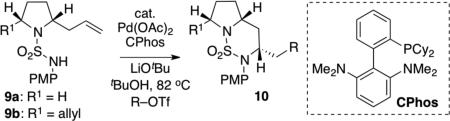
| |||||
|---|---|---|---|---|---|
| fileentry | R1 | R | product | yield (%)b | drc (crude) |
| 1 | H | Ph | 10a | 89 | 7:1 |
| 2 | H | p-tBu-C6H4 | 10b | 78 | 6:1 |
| 3 | H | p-MeO-C6H4 | 10c | 70 | 7:1 |
| 4 | H | p-benzophenone | 10d | 61d | 8:1 (5:1) |
| 5 | H | o-Me-C6H4 | 10e | 87 | 5:1 |
| 6 | H | 1-cyclohexenyl | 10f | 63d | 6:1 |
| 7 | H | 1-decenyl | 10g | 45d | 10:1[f,g] |
| 8 | allyl | Ph | 10h | 65e | 20:1 (12:1) |
| 9 | allyl | P-MeO-C6H4 | 10i | 63e | >20:1 (13:1) |
Reaction Conditions: 1.0 equiv 9a or 9b, 2.0 equiv R-OTf, 2.0 equiv LiOtBu, 4 mol % Pd(OAc)2, 10 mol % C-Phos, tBuOH (0.1 M), 82 °C, 16 h.
Isolated yield (average of two or more runs).
Diastereomeric ratio of the pure isolated material. Diastereomeric ratios of isolated materials were identical to those of the crude products unless otherwise noted.
The reaction was conducted with 3.0 equiv of LiOtBu and 3.0 equiv R-OTf.
The reaction time was 2 h.
1-Decenyl triflate was employed as 5:1 mixture of E:Z isomers.
The dr was determined following hydrogenation of 10g. The crude dr of 10g could not be determined directly due to the mixture of diastereomers and E/Z isomer products. However, we estimate the crude dr to be ca. 5-10:1.
