Abstract
Background
To study prolonged subcutaneous dosing of systemic 852A, a Toll-like receptor-7 agonist (TLR-7), in recurrent breast, ovarian and cervix cancer to assess anti-tumor activity. Secondary objectives included assessment of safety and immune system activation.
Methods
Adults with recurrent breast, ovarian or cervix cancer failing multiple therapies received 0.6 mg/m2 of 852A subcutaneously twice weekly for 12 weeks. Doses increased by 0.2 mg/m2/week to a maximum of 1.2 mg/m2. Serum was collected to assess immune activation.
Results
Fifteen patients enrolled: 10 ovarian, 2 cervix and 3 breast. Three completed all 24 injections. There were two grade 2 (decreased ejection fractions), nine grade 3 (1 cardiovascular, 1 anorexia, 3 dehydration, 2 infections, 2 renal) and two grade 4 (hepatic and troponin elevation) unanticipated toxicities. Cardiac toxicities included three cardiomyopathies (two asymptomatic) and one stress-related non-ST elevated myocardial infarction. Five patients discontinued therapy due to possibly associated side effects. One had stable disease (SD) following 24 doses and received 17 additional doses. A cervix patient had SD following 24 doses, progressed 3 months later, received chemotherapy and remains disease free at 18 months. Immune activation, as evidenced by increased IP-10 and IL-1ra, was observed.
Conclusions
In this first human experience of a TLR-7 agonist delivered subcutaneously using a prolonged dosing schedule 852A demonstrated sustained tolerability in some patients. Clinical benefit was modest but immune activation was seen suggesting further study of antitumor applications is warranted. Because of cardiac toxicity; treatment with 852A should be used cautiously in heavily pretreated patients.
Keywords: TLR-7 agonist, 852A, ovarian cancer, cervix cancer, breast cancer
Introduction
Although the immune system can control carcinogenesis [1], our success with immunotherapy is limited in patients with established tumor in part because of presumed inadequate activation of a coordinated response between the innate and adaptive immune system. Infectious disease components containing anti-tumor activity have been identified as pathogen associated molecular patterns (PAMPs) which activate the innate immune system through toll-like receptors (TLRs). TLRs are preferentially expressed on cells of the innate immune system, including dendritic cells (DC)[2]. Engagement of TLRs on DC’s promotes cross-talk between the innate and the adaptive immune system, immune cell maturation, and migration of DCs into lymph nodes leading to activation, proliferation and survival of tumor antigen-specific CD4+ and CD8+ T cells. Since tumor cells usually do not express molecules which induce DC maturation, the application of TLR agonists is an important step in activating T cells.
Imidazoquinolines (including imiquimod and resiquimod [R-848]), are low molecular weight synthetic compounds that have potent immune stimulating properties and have demonstrated anti-viral and anti-tumor activity.[3-5] Molecule 852A (also known as S-32865, is N-[4-(4-amino-2-ethyl-1H-imidazo[4,5-c]quinolin-1-yl)butyl]methanesulfonamide) is a novel immune response modifier (IRM), related to the imidazoquinoline molecule imiquimod, that acts as a TLR7 agonist. Imiquimod, brand name Aldara 5% cream™, has demonstrated antiviral and antitumor activity when topically applied to external genital and perianal warts, superficial basal cell carcinoma, actinic keratosis and metastases from malignant melanoma. The mechanism of immune activation by imidazoquinolines is via recognition by TLR7 or TLR 8 and activation of a MyD88-dependent pathway. MyD88 is a Toll-interleukin 1 receptor domain-containing protein which recruits the IL-1 receptor-associated kinase (RIAK) and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) to the TLR, which ultimately activates NF-kB transcription factors[6]. Imiquimod induces type I interferon (IFN) signaling[7] and has been shown to induce apoptosis in tissue culture.[8]
The first human phase I trial of 852A was in patients with refractory solid organ tumors[9]. Drug was delivered IV and was limited to 6 doses, a duration too short to assess the response of the TLR-7 agonist. Recently 852A’s bioavailability, dose proportionality and tolerability were evaluated in a Phase I study enrolling healthy volunteers[10]. Subjects received 852A by the intravenous (IV), subcutaneous and oral (PO) routes. Absolute bioavailability for subcutaneous delivery determined over a 24-hour period was approximately 80% and pharmacokinetic data for Cmax and AUC(0-t) demonstrated dose proportionality for subcutaneous delivery. Therefore, we chose to test 852A subcutaneous delivery in refractory breast, ovarian and cervical malignancies to minimize patient burden and to allow for self-administration to patients who tolerated the drug for an extended period of time.
Materials and Methods
Study Criteria
We conducted a prospective, phase II trial at the University Of Minnesota-Fairview Medical Center. Subjects were recruited between 8/06-11/07 following Institutional Review Board approval and in accordance with the ethical standards in the 1964 Declaration of Helsinki. Female patients age >18, were eligible for enrollment if they had recurrent epithelial ovarian, fallopian tube or primary peritoneal disease or recurrent/persistent squamous or adenocarcinoma of the cervix failing two or more prior therapies. Breast cancer patients were enrolled if they had failed at least two previous chemotherapy or hormonal regimens for metastatic disease. Patients had to have had measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.0 with at least one target lesion > 10 mm in diameter, GOG performance score ≤ 2 or Karnofsky performance status ≥ 50 (breast). All patients had adequate organ function, a platelet count ≥ 50,000/ul, a hemoglobin level ≥ 9gm/dL, and an absolute neutrophil count ≥1000/ul. Drugs known to induce QT interval prolongation and/or induce Torsades De Pointes were not allowed.
Treatment
Following informed consent, 852A (0.2% solution; 3M pharmaceuticals, St. Paul MN) was administered subcutaneously twice weekly for 12 weeks (24 doses). The starting dose for all subjects was 0.6 mg/m2. If no dose limiting toxicity (described under toxicity assessments) or other symptoms of intolerance were observed after at least two consecutive injections, 852A was increased by 0.2 mg/m2 increments to a maximum of 1.2 mg/m2. BSA was calculated only once per course using the Dubois method. Patients with at least stable disease were allowed to receive additional 12-week treatment cycles.
Clinical assessments
Echo or Multi Gated Acquisition Scan (MUGA) scan was performed at baseline and at the end of therapy. Cardiac troponin was assessed at baseline and monthly. Clinical response was determined by RECIST Version 1.0 based on tumor evaluation done at baseline and on completion of treatment at week 13 or earlier using computed tomography (CT), positron emission tomography (PET) and or magnetic resonance imaging scans (MRI).
Toxicity assessments
Toxicities were graded according to the National Cancer Institute (NCI) CTCAE Version 3. Toxicity assessments were performed within one month prior to dose 1, weekly until the patient reached their maximum tolerated dose and then every 2 weeks. Dose limiting toxicities were any grade 3 or 4 non-hematologic toxicity with the following exceptions: constitutional symptoms (transient chills, myalgias, arthralgias, headache, anorexia), nausea and vomiting controlled with anti-emetics, grade 3 fever, and grade 4 fatigue for < 24 hours. Hematological dose limiting toxicities included ANC<1000 after 3 doses of G-CSF and thrombocytopenia to < 30,000.
Immune monitoring
Immunologic monitoring included serum cytokine measurement from blood obtained pre- and 6 hours post-dose for dose 1, and for some patients mid therapy (between doses 10-14), and at final dosage (dose 24). Serum cytokine/chemokine levels were determined by multiplex analysis on the Luminex platform (Austin, TX) using a 22-plex (Human cytokine panel A) from R&D systems (Minneapolis, MN) and a 7-plex panel (for sCD40L, IL-12p70, IL-7, IL-12p40, IL-13, IL-15 and IP-10/CXCL10) from Millipore-Linco (Billerica, MA). Frequency and phenotype of lymphocyte populations were determined by flow cytometry analysis using methods previously described [9]
Statistics
Subjects had to receive at least 12 doses of 852A to be evaluable for clinical efficacy. Sample size was estimated prospectively to have less than 5% type I error and 80% statistical power to detect a response rate of at least 30% in a two-stage design. The change in cytokines before and after treatment was tested using the Wilcoxon Signed Rank test. All analyses were performed using SAS version 9.1.
Results
Patient characteristics and clinical response
Fifteen patients were enrolled during the 15 month accrual period and 14 received treatment: 9 ovarian, 2 cervix and 3 breast (Table 1). The median number of prior chemotherapy regimens was 6.5 (range: 2-16). Median age was 53 years (range: 25-66), mean weight was 76.7 kg (range: 43-117), 12 (86%) were Caucasian, and 2 (14%) black.
Table 1.
Demographics and tumor characteristics
| Characteristic (N=14) | |||
|---|---|---|---|
| Age Group, years (%) | 18-29 | 1 | (7) |
| 30-39 | 0 | (0) | |
| 40-49 | 2 | (14) | |
| 50-59 | 9 | (64) | |
| 60-69 | 2 | (14) | |
| Age, median years (range) | 53 | (25- 66) |
|
| Race (%) | Caucasian | 12 | (85) |
| Black | 2 | (14) | |
| Weight (kg), mean (range) | 76.7 | (43- 117) |
|
| Primary Site (%) | Ovary | 9 | (64) |
| Cervix | 2 | (14) | |
| Breast | 3 | (21) | |
| GOG Performance Status (%) | 0 | 9 | (64) |
| 1 | 3 | (21) | |
| 2 | 2 | (14) | |
| Prior treatment regimens, median (range) | 6.5 | (2-16) | |
| Median doses received (range) | 12 | (0-37) | |
| Received max dose of 1.2 mg/m2, (%) | 9 | (64) | |
| Total evaluable patients | Cervix | 2 | |
| Ovary | 5 | ||
| Breast | 0 |
There were 7 evaluable patients who received at least 12 doses of 852A: 2 patients with cervix cancer and 5 with ovarian cancer. The median number of doses received was 12 (range 1-37). The reasons for discontinuation of treatment are listed in Table 2. Four patients completed all 24 injections, one of whom had stable disease following 24 doses, recurred 3 months after completing therapy, received chemotherapy and remains disease free 18 months later. One of the patients who had stable disease following 24 doses received 13 additional doses at 1.2 mg/m2 until she developed anorexia, nausea and vomiting and discontinued therapy. Three had at least 12 injections but had disease progression causing them to discontinue drug before dose 24. Another ovarian cancer patient had stable disease following 10 injections but was taken off study due to fatigue.
Table 2.
852A dose administration
| Patient | Total Doses Received |
Max Dose Received |
# of Max doses Received |
Dose Reduction |
Treatment Discontinued |
Reason |
|---|---|---|---|---|---|---|
| 1 | 24 | 1.2 | 14 | no | no | |
| 2 | 12 | 1.2 | 5 | yes | yes | disease progression |
| 3 | 9 | 1.2 | 3 | no | yes | disease progression |
| 4 | 6 | 1 | 2 | no | yes | nausea/vomiting |
| 5 | 1 | 0.6 | 1 | no | yes | EF-15% |
| 6 | 10 | 1.2 | 4 | no | yes | fatigue |
| 7 | 5 | 1 | 1 | no | yes | disease progression |
| 8 | 24 | 1.2 | 18 | no | no | |
| 9 | 12 | 1.2 | 6 | no | yes | disease progression |
| 10 | 37 | 1.2 | 31 | no | during second course | anorexia, nausea/vomiting |
| 11 | 24 | 1.2 | 2 | yes | no | |
| 12 | 2 | 0.6 | 2 | no | yes | non-ST elevated MI |
| 13 | 14 | 1.2 | 8 | no | yes | disease progression |
| 14 | 0 | DOD before first dose |
DOD=Dead of Disease
Toxicity
Unanticipated toxicities are summarized in Table 3. Of 14 patients enrolled in this study, there were no grade 1 toxicities, 2 grade 2 toxicity (left ventricular dysfunction), 9 grade 3 (1 cardiovascular, 1 anorexia, 3 dehydration, 2 infections, and 2 renal) and 2 grade 4 toxicities (hepatic and troponin elevation). Table 4 demonstrates the maximum grade targeted toxicity experienced for each patient in the trial. Pain was a commonly reported toxicity, with 4 patients experiencing grade 3 pain, however all events were thought to be attributable to disease. There were 12 patients who experienced malaise and 11 who experienced nausea during the trial. Five patients discontinued therapy due to side effects possibly associated with 852A: one due to decreased ejection fraction, one due to a non ST elevated myocardial infarction, two due to nausea and vomiting, and the other one due to fatigue. Cardiac toxicity emerged as a particular unexpected concern and the two events considered DLT and two asymptomatic patients identified during cardiac follow-up are described below in detail.
Table 3.
Summary of unanticipated drug-related adverse events
| CTCAE grade |
||||
|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 |
| Breast | ||||
| Cardiovascular | 0 | 0 | 1 | 0 |
| TOTAL | 0 | 0 | 1 | 0 |
| Ovarian | ||||
| Troponin elevation | 0 | 0 | 0 | 1 |
| Left ventricular function | 0 | 2 | 0 | 0 |
| Anorexia | 0 | 0 | 1 | 0 |
| Dehydration | 0 | 0 | 2 | 0 |
| Elevated liver enzymes | 0 | 0 | 0 | 1 |
| Infection | 0 | 0 | 1 | 0 |
| Renal Failure | 0 | 0 | 1 | 0 |
| TOTAL | 0 | 2 | 5 | 2 |
| Cervix | ||||
| Dehydration | 0 | 0 | 1 | 0 |
| Infection | 0 | 0 | 1 | 0 |
| Renal | 0 | 0 | 1 | 0 |
| TOTAL | 0 | 0 | 3 | 0 |
| TOTAL grade 3 and 4 toxicity | 0 | 2 | 9 | 2 |
CTCAE: Common Terminology Criteria for Adverse Events
Table 4.
Maximum grade targeted toxicity
| Category | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Nausea | 6 | 5 | 0 | 0 |
| Vomiting | 1 | 5 | 0 | 0 |
| Dyspnea | 4 | 3 | 2 | 0 |
| Fever | 3 | 2 | 2 | 0 |
| Chills | 5 | 4 | 0 | 0 |
| Myalgia | 3 | 2 | 1 | 0 |
| Sweats | 5 | 2 | 0 | 0 |
| Malaise | 3 | 6 | 3 | 0 |
| Edema | 5 | 1 | 0 | 0 |
| Cough | 6 | 2 | 0 | 0 |
| Pain | 1 | 2 | 4 | 0 |
| Total | 42 | 34 | 12 | 0 |
Patient 1 was a 45 year old female diagnosed with an estrogen receptor positive breast cancer who developed grade 3 cardiomyopathy following her first 852A injection. At age 34 she underwent mastectomy followed by adjuvant chemotherapy including doxorubicin. She had multiple recurrences and treatments which included additional anthracyclines. At study entry the patient had metastatic lesions to the spine and liver. The patient had a MUGA scan prior to trial initiation that showed a left ventricular ejection fraction (LVEF) of 43%. Twelve hours following her first injection, she developed sudden substernal chest pain with significant shortness of breath, cough, vomiting and diarrhea during the night. Troponin was elevated at 0.56 without EKG changes, D-Dimer was elevated at 3.1 and B-type natriuretic peptide was elevated at 3020. Transthoracic echo demonstrated severely decreased LV function with an estimated ejection fraction of 15%. The patient was managed with lasix, a beta blocker, and an ACE inhibitor. Fourteen days later her ejection fraction improved to 40%. By 2 months her ejection fraction was 53%. This patient’s 6 hour post dosing IP-10, IL-1ra, and MCP-1cytokine levels rose significantly from 235.1 to 5045.2 pg/ml, 624 to 20,151.3 pg/ml, and 257 to 1696 pg/ml, respectively. The increases in IP-10 and IL-1ra were the second largest observed among this cohort of patients.
Patient 2 was a 47 year old woman with platinum resistant Stage IIIc ovarian carcinoma who received adjuvant paclitaxel and carboplatin, consolidation with docetaxel followed by liposomal doxorubicin. Her pre study MUGA was 72%. Two days prior to her first injection, the patient had diarrhea and had received IV fluids for dehydration. Two days following her first injection, she was admitted to the hospital with dehydration, acute renal failure, and weakness. Troponin on admission was 4.33 ug/L. During her hospitalization her creatinine rose to 3.44 mg/dl, ALT =140 u/l and AST =736 u/l, uric acid=12 mg/dl, phosphorus was as high as 6.3 mg/dl and calcium decreased to 6.9 mg/dl. ECG revealed sinus tachycardia and the patient was admitted to cardiology with diagnosis of a non-ST elevation myocardial infarction (MI) and possible tumor lysis syndrome. MUGA was repeated and was 80%. Patient was discharged on hospital day 10 with resolution of her lab abnormalities. This patient’s 6 hour post dosing IP-10, IL-1ra, and MCP-1cytokine levels rose significantly from 1017.5 to 5119.6 pg/ml, 3145.2 to > 42,000 pg/ml and 189.9 to 6185.8 pg/ml, respectively.
Patient 3 was a 54 year old diagnosed with Stage IIIc serous ovarian cancer who received adjuvant paclitaxel and carboplatin followed by altretamine consolidation. Following recurrence she went on to receive topotecan followed by liposomal doxorubicin, gemcitabine, cisplatin, and paclitaxel. Prior to beginning the 852A study her LVEF was 51%. Following completion of 24 doses this decreased to 42.6%. She was managed with an ACE inhibitor and a beta blocker. Three months later her LVEF was 53%. The patient died of progressive disease 4 months later.
Patient 4 was a 52 year old patient diagnosed with stage IIIC serous ovarian cancer who, after receiving adjuvant paclitaxel and carboplatin, recurred within 3 months and started liposomal doxorubicin. She went on to receive: gemcitabine, topotecan, 5-FU plus oxaliplatin plus leucovorin, cyclophosphamide, etoposide and doxorubicin. She received her last dose of doxorubicin three weeks prior to starting 852A. Her pre study LVEF was 68%. Due to the previously observed cardiotoxicity, a MUGA was obtained 2 months after initiating treatment, following her 16th injection. Her LVEF decreased to 57% and subsequently to 48% following her 24th injection. The patient remained without evidence of heart failure and had stable disease. She desired a second course of TLR-7 agonist. One month following completion of her first 24 doses, a repeat MUGA showed a LVEF of 60% and the patient elected to start a second course of therapy. She received 17 injections and discontinued the study due to disease progression during which she did not experience any additional cardiac issues.
Immunologic effects of 852A
We evaluated a panel of cytokines thought to be involved in the dendritic cell NK pathway. There was a statistically significant increase in IL-1ra and IP-10, with a 2.6 and 4.9 fold change, respectively observed from baseline to 6 hours following the first dose of 852A (Table 5). The increase in IL-1ra and IP-10 levels were maintained after repeated doses with no evidence of diminished responsiveness (Figure 1). Based on our results, increases in cytokine measurements are short lived and there was no evidence of cytokine accumulation, but responsiveness was maintained mid-therapy at levels similar to initial dosing. Lymphocyte subsets were evaluated at baseline and 6 hours after the first dose of 852A to determine if NK cell activation occurred. No lymphocyte subsets changed more than a median of 2 fold. For a subset of patients (4/13) CD69, a marker of NK cell activation, was significantly increased 6 hours after the first dose of 852A compared to baseline levels (data not shown). There was no correlation between NK cell activation and IL-10 and IL-1ra levels.
Table 5.
Cytokine median values and ranges pre-treatment and 6 hours post-treatment dose 1
| Cytokine | N | Median (Range) |
Median fold
chg(Range) |
p-value |
|---|---|---|---|---|
| IL-1ra | ||||
| Pre-treatment | 13 | 903.30 (420.37, 4591.80) | ||
| 6 hrs after treatment | 12 | 2981.15 (505.29, 42000) | ||
| DIFFERENCE | 12 | 1902.16 (−60.67, 38854.80) |
2.6 (0.95, 32.3) | 0.0024* |
| IP-10 | ||||
| Pre-treatment | 13 | 210.60 (71.44, 1017.52) | ||
| 6 hrs after treatment | 12 | 1129.79 (176.12, 5164.90) | ||
| DIFFERENCE | 12 | 856.84 (34.53, 4810.08) | 4.9 (1.1, 21.5) | 0.0005* |
IL -1α, IL -1β, IL - 1ra, IL -2, IL -4, IL - 5, IL - 6, IL -8, IL -10, IL -17, ENA −78/CXCL5, FGF -basic, G-CSF, GM -CSF, IFN -γ, MCP -1/CCL2 ,MIP -lα,/CCL3 MIP -lβ/CCL4 , RANTE S/CCL5 , TNF - α, Tpo, YEGF , sCD40L, IL -12p70, IL -7, IL -12p40, IL -13, IL -15 and IP -10/CXCL10 were analyzed. The table represents the cytokines that were significantly different between pre and 6 hours post 852A dose 1.
Figure 1.
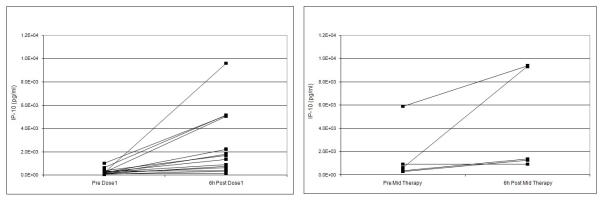
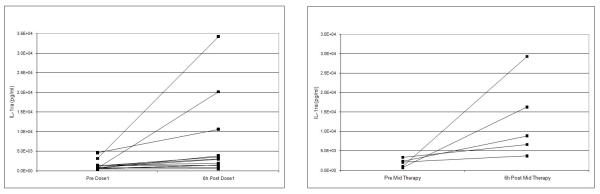
IP-10 levels (pg/ml) (panel A) and IL-1ra levels (pg/ml) (panel B) prior to 852A injection and 6 hours post injection at dose 1 and at mid therapy dose.
Discussion
852A can be delivered subcutaneously using a prolonged dosing schedule up to 1.2 mg/m2 twice weekly. Evidence of clinical and serological immune activation was present in our advanced disease cohort. Although all toxicities attributed to the study drug were reversible the cardiotoxic events were unexpected and warrant cautious use in heavily pretreated patients. The majority of patients experienced malaise a side effect that has been associated with other TLR-agonists.
852A directly stimulates cells in the innate immune system, via the TLR7 pathway, resulting in dendritic cell maturation, induction of multiple cytokines including IFN-α, and enhanced antigen presentation[11], which makes 852A desirable for therapeutic application. Dudek and colleagues established the maximum tolerated IV dose of 1.2 mg/m2 of 852A three times weekly in their phase I study[9]. Similar to our study using subcutaneous dosing, several studies observed increases in IP-10 and IL-1ra biomarkers following dosing of at least 0.6 mg/m2. [9, 12]. Although levels of IP-10 and IL-1ra rose six hours following the first injection and after repeat injections, levels did not continually rise. IP-10 has been shown to promote induction of T helper 1 cytokines necessary for antitumor response and represents a downstream marker for the presence of IFN-α, a biomarker associated with TLR-7 agonists[13-15]. Although not statistically elevated following injection, like IP-10, MCP-1 has been shown to augment NK cell cytolysis in vitro and induce potent NK cell migration[16]. Changes in the activation markers (IP-10 and IL-1ra) associated with TLR-7 agonists support the proposed pharmacologic activity of 852A as a selective TLR7 agonist in agreement with the findings of others[9]. Although we did not see any correlation between increased IP-10 and IL-1ra levels with NK cell activation, it is possible that this measure is confounded by decreases in NK cells sampled from blood as a result of the activation process and egress of lymphocytes from blood into tisse. We acknowledge that monitoring peripheral blood may not accurately portray NK cell activation and that tissue sampling may better represent the state of NK cells following an immune modulating drug such as 852A. Trafficking studies will be an important adjunct to future studies.
The tumors we focused on in this study had potential for immune responses. Breast cancers are immunogenic tumors and most patients demonstrate cellular and humoral immune responses [17]. A recently published report of an off-label use of Imiquimod (Aldara) to treat cutaneous breast cancer metastases described two women who had complete resolution of their cutaneous disease after topical application of the imidazoquinoline cream[18]. We chose to study 852A in an ovarian cancer population given a history of strategies testing immune responses, including the use of other TLR agonists and tumor-derived exosomes carrying tumor-associated antigens, in advanced ovarian cancer patients[19]. The cervix cancer population is an obvious population to examine 852A in as more than 90% of cervix cancer cases are now known to be the result of infection with high-risk HPV types such as 16 and 18. Imiquimod (Aldara), another TLR7 agonist, has shown efficacy in the treatment of external genital warts often caused by HPV 6 and 11 as well as recurrent high-grade intraepithelial neoplasia of vulva, vagina and cervix [20].
The observed cardiac adverse events associated with 852A administration have not previously been reported. A known potential side effect of 852A is prolongation of action potential in hERG potassium ion channels. This prolongation has been observed in vitro at 3,000 ng/ml of 852A with QTc increases in dogs at 2.5 mg/kg[21]. No adverse trends in electrocardiogram QTc measurements were reported by Dudek or Dummer[9, 21]. The decreases in LVEF and non-ST elevation myocardial infarction seen in our cohort have not previously been reported with 852A. The patient with the most extreme cardiomyopathy had received doxorubicin 10 years prior to the study and, among multiple other regimens, had received two additional courses of liposomal doxorubicin one year prior to study entry and again one month before study entry. Six hours following her first 852A injection, this patient had large increases in her serum cytokine levels. Her IP-10 level was 21 times, IL-1ra 32 times, and MCP-1 6.6 times that of baseline. The observed decrease in ejection fraction followed by resolution within 3 months may be a result of acute immune activation and cytokine storm after a single 852A dose. Similarly, the ovarian cancer patient who experienced the non-ST elevation MI in the setting of tumor lysis had significant increases in her cytokine levels after 852A dosing. Further investigation is warranted to characterize the immune response seen in patients who developed cardiotoxic events. The significant increase in cytokines observed in the two patients described above was not seen in the ovarian cancer patients who experienced transient asymptomatic decreases in their ejection fraction.
Although a true efficacy rate could not be determined, a key clinical finding was disease stabilization observed in 2 heavily pretreated patients, one of whom was an ovarian cancer patient. This woman had platinum refractory disease and had received 8 prior therapies before starting 852A. Following 24 doses of the drug, she had stable disease and went on to receive thirteen additional doses before disease progression. The other patient with stable disease had Stage IIIB squamous cell cervix cancer and had received radiation and 2 prior chemotherapy regimens. She was found to have stable disease following twenty-four doses of drug but progressed 3 months following her last injection. At the time of her progression she was placed on chemotherapy and is without evidence of disease 18 months later. This certainly is an unusual case of recurrent cervix cancer where response rates for recurrent disease in patients with recurrent disease or pelvic metastases have a poor prognosis with 1-year survival rates between 15% and 20%[22]. This patient is currently 27 months from trial completion which begs the question of whether TLR-7 agonists, as initiators of innate and adaptive immune responses, could have “primed” the tumor cells for the next therapy resulting in the unlikely effective antitumor response observed. The stable disease observed following use of this drug, led us to believe that combination therapy would be of potential interest for future phase II studies.
In summary, we report the first subcutaneous administration of 852A, a selective small-molecule TLR-7 agonist, in patients with recurrent ovarian, cervix, and breast cancers. Grade 3 and 4 toxicities were short lived and manageable. The frequency and severity of cardiac toxicity, while apparently reversible, exceeded expectations and may limit the development of this agent. However, we did find evidence of immune activation and disease stabilization in 2 patients suggesting that 852A may have a role for future systemic antitumor studies. Immunotherapy alone is unlikely to have a major impact on a disease associated with bulky tumor as is frequently the situation in recurrent solid tumors. The limited efficacy we observed with 852A monotherapy in our heavily pre-treated, advanced disease population is not surprising. Optimizing this therapy may require integration into front line treatment or as a vaccine adjuvant in order to maximize T-cell and NK activation. Additional preclinical and clinical studies would be necessary to clarify what role TLR7 agonists could play as antitumor treatments.
Acknowledgement
This study was initially supported by 3M. 3M oncology programs were acquired by Coley in June 2007 and Coley provided financial support for MUGA scans performed as part of the trial. Coley was acquired by Pfizer Inc in November 2007. Additional support was provided by the Minnesota Ovarian Cancer Alliance.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 2.Kokkinopoulos I, Jordan WJ, Ritter MA. Toll-like receptor mRNA expression patterns in human dendritic cells and monocytes. Mol Immunol. 2005;42:957–968. doi: 10.1016/j.molimm.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Miller RL, Gerster JF, Owens ML, et al. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Griffith BP, Lucia HL, Hsiung GD. Efficacy of S26308 against guinea pig cytomegalovirus infection. Antimicrob Agents Chemother. 1988;32:678–683. doi: 10.1128/aac.32.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein DI, Harrison CJ, Tomai MA, Miller RL. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J Infect Dis. 2001;183:844–849. doi: 10.1086/319262. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel J, Uerlich M, Haller O, et al. Enhanced type I interferon signaling and recruitment of chemokine receptor CXCR3-expressing lymphocytes into the skin following treatment with the TLR7-agonist imiquimod. J Cutan Pathol. 2005;32:257–262. doi: 10.1111/j.0303-6987.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T, Nindl I, Schmook T, et al. Induction of apoptosis by Toll-like receptor-7 agonist in tissue cultures. Br J Dermatol. 2003;149(Suppl 66):9–14. doi: 10.1046/j.0366-077x.2003.05632.x. [DOI] [PubMed] [Google Scholar]
- 9.Dudek AZ, YC, Harrison LI, Kumar S, Hawkinson R, et al. First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clinical Cancer Research. 2007;13:7119–7125. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LI, Astry C, Kumar S, Yunis C. Pharmacokinetics of 852A, an imidazoquinoline Toll-like receptor 7-specific agonist, following intravenous, subcutaneous, and oral administrations in humans. J Clin Pharmacol. 2007;47:962–969. doi: 10.1177/0091270007303766. [DOI] [PubMed] [Google Scholar]
- 11.Schiller M, Metze D, Luger TA, et al. Immune response modifiers--mode of action. Exp Dermatol. 2006;15:331–341. doi: 10.1111/j.0906-6705.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 13.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. Faseb J. 1998;12:705–713. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 14.Yoneyama H, Narumi S, Zhang Y, et al. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 16.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 17.Disis ML. Immunologic targets for breast cancer. Breast Dis. 2002;15:83–90. doi: 10.3233/bd-2002-15109. [DOI] [PubMed] [Google Scholar]
- 18.Hengge UR, Roth S, Tannapfel A. Topical imiquimod to treat recurrent breast cancer. Breast Cancer Res Treat. 2005;94:93–94. doi: 10.1007/s10549-005-7017-2. [DOI] [PubMed] [Google Scholar]
- 19.Adams M, Navabi H, Croston D, et al. The rationale for combined chemo/immunotherapy using a Toll-like receptor 3 (TLR3) agonist and tumour-derived exosomes in advanced ovarian cancer. Vaccine. 2005;23:2374–2378. doi: 10.1016/j.vaccine.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Arrastia C, Arany I, Robazetti SC, et al. Clinical and molecular responses in high-grade intraepithelial neoplasia treated with topical imiquimod 5% Clin Cancer Res. 2001;7:3031–3033. [PubMed] [Google Scholar]
- 21.Dummer R, Hauschild A, Becker JC, et al. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res. 2008;14:856–864. doi: 10.1158/1078-0432.CCR-07-1938. [DOI] [PubMed] [Google Scholar]
- 22.Bonomi P, Blessing JA, Stehman FB, et al. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079–1085. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]


