Fig. 2.
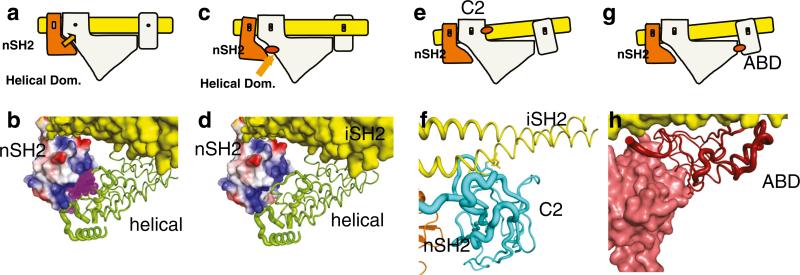
Schematic representation of the mechanism of activation by phosphorylated RTK peptides and by oncogenic mutations at domain interfaces. a Wild-type resting PI3K. Orange arrow indicates inhibition by nSH2. b Binding of phosphorylated peptide (purple) disrupts the inhibitory interaction as shown diagramatically in (c). d Mutation in the helical domain have the same effect as phosphorylated binding shown in (c and e). e Mutations in the C2 domain of p110α change the C2/iSH2 interaction resulting in a weakening of the inhibitory interaction of the nSH2 with the helical domain. g, h ABD mutations in the ABD/Kinase domain affect the ABD/iSH2 interaction at one end, which in turn lessens the nSH2/ helical domain interaction