Abstract
Viable new treatments for depression and anxiety have been slow to emerge, likely owing to the complex and incompletely understood etiology of these disorders. A budding area of research with great therapeutic promise involves the study of resilience, the adaptive maintenance of normal physiology and behavior despite exposure to marked psychological stress. This phenomenon, documented in both humans and animal models, involves coordinated biological mechanisms in numerous bodily systems, both peripheral and central. In this review, we provide an overview of resilience mechanisms throughout the body, discussing current research in animal models investigating the roles of the neuroendocrine, immune, and central nervous systems in behavioral resilience to stress.
Keywords: resilience, stress, depression, anxiety, neuroimmune, plasticity
1.0 Introduction
Decades of research on human stress resilience have followed its initial description in at risk children in the 1970s [1]. Resilience is defined as the adaptive maintenance of normal physiology, development and behavior in the face of pronounced stress and adversity. Human resilience literature contains countless examples of adults and children who, despite marked psychological stress, display minimal adverse changes in emotional wellbeing or behavioral disturbances [2-6]. Although it is tempting to attribute human resilience to the possession of exceptional abilities and coping mechanisms, both social and biological, most people do not develop anxiety and depression when faced with stress [1, 6]. Resilience is a common outcome that more likely involves the successful application of the body's adaptive stress response to maintaining the status quo. The biological processes underlying resilience are often collectively termed “allostasis” and constitute variation in bodily systems that functions to maintain homeostasis in response to a stressor [7]. In some cases, allostasis is exaggerated or fails to cease along with the stressor, and mechanisms that were once protective can become pathological. This phenomenon—termed “allostatic load”—can potentially result in physiological and psychological damage, including enhanced susceptibility to disorders such as depression and anxiety [7, 8].
Mechanisms of resilience are of great interest due to the serious burdens imposed on patients and society by stress-related disorders including anxiety and depression. One in six Americans will develop Major Depressive Disorder (MDD) during their lifetime, a particularly alarming statistic as only 30% of patients achieve complete remission of symptoms following treatment with current first-line therapies, the monoamine-based antidepressants [9, 10]. When not adequately treated, MDD can become a chronic, recurrent condition characterized by escalating disability [11]. Comprehensive knowledge of the etiology of depression is still lacking. Understanding the adaptive, allostatic mechanisms that protect most individuals against psychopathology can potentially inform therapeutic development and treatment strategies for more vulnerable individuals.
Depression and anxiety are increasingly considered to be “whole body” illnesses involving the dysregulation of multiple systems, both peripheral and central. Similarly, resilience likely results from successful allostatic mechanisms in the hypothalamo-pituitary-adrenal (HPA) axis, autonomic nervous system, immune system and the brain [7]. In this review, we summarize recent research into the roles of the neuroendocrine, immune and central nervous systems in resilience to stress, focusing primarily on animal models. We describe both active, compensatory mechanisms as well as passive mechanisms in which the absence of a maladaptive stress response promotes resilience. We present resilience as an integrated process by which adaptive mechanisms in multiple systems promote psychological resilience to stress.
2.0 Animal Models for the Study of Stress Vulnerability and Resilience
Research on human subjects has yielded important insights into the roles of various neurotransmitters, neuropeptides and hormones as well as genetic factors in the neurobiology of resilience (for comprehensive reviews, see [8] and [12]). For ethical and practical reasons, animal models are often employed to examine the causative effects of stress on biological processes in the brain and body. Resilience to stress has been documented and characterized in animal models throughout the lifespan. Below, we describe in detail several behavioral paradigms commonly used to elicit and study stress resilient phenotypes in juvenile and adult animals.
2.1 Early Life Stress
Models of early life stress have informed our understanding of a form of resilience called stress inoculation, whereby early stressful experience attenuates stress response in adulthood. In children, early stress can have a “steeling” effect, promoting subsequent stress resistance and successful psychological functioning [13]. Animal models of early life stress typically involve exposure to stressful stimuli during either the prenatal or postnatal periods. Prenatal stressors include maternal stress such as glucocorticoid administration or food deprivation while early postnatal stressors include brief bouts of maternal separation, altered maternal care behavior, or glucocorticoid administration [14]. Prolonged early life stress can cause programmed HPA axis overactivity, altered glucocorticoid response, structural changes in the brain, and deleterious effects on cognition, emotion and behavior [14]. These effects can be reconciled with the concept of stress inoculation by imagining adult outcomes of early life stress as a U-shaped curve—animals exposed to moderate stress in early life show better outcomes and more adaptive responses to stress in adulthood than do animals exposed to minimal or severe stress [15]. Stress inoculation has been demonstrated in both primates and rodents. Infant squirrel monkeys separated from their mothers for brief, intermittent periods demonstrate reduced hormonal stress response in subsequent developmental stages [16, 17]. They also demonstrate cognitive and emotional resilience across measures relevant to anxiety and depression, such as enhanced novelty tolerance, exploratory behavior and behavioral response inhibition [16-18]. There is a rich literature on stress inoculation in rodents demonstrating that rats exposed to early life stress, including brief maternal separations and neonatal corticosterone administration, display blunted HPA axis response to stress in adulthood as well as behavioral resilience in the form of reduced anxiety-like behavior and enhanced performance in cognitive tasks [19-23]. The relationship between early life stress exposure and subsequent resilience in both primates and rodents follows the abovementioned U-shaped curve. Prolonged maternal separation and social isolation in infant rhesus monkeys produces an increased stress response and “despair-like” behavior in subsequent social separation tests [24]. Rats exposed to moderate early life stress show enhanced measures of resilience compared to both severely and minimally stressed rats [21]. For example, early postnatal rats exposed to brief daily handling (a moderate stressor) subsequently show attenuated stress response compared to undisturbed pups and pups exposed to prolonged daily maternal separation (a more severe stressor) [20, 25].
2.2 Chronic Unpredictable Stress
Chronic Unpredictable Stress (CUS) is a useful model for examining stress vulnerability and resilience in rodents [26, 27]. In CUS paradigms, animals are exposed to varying mild stressors sequentially for a period of 1-7 weeks [28, 29]. Stressors can include mild foot shock, physical restraint, tail suspension, light/dark cycle disruption, food or water restriction, changes to cage mate, etc., and are changed after several hours to minimize habituation [27, 29]. CUS produces a range of depression and anxiety-like behaviors in rodents including anhedonia, measured as decreased sucrose preference, despair-like behavior, measured as increased immobility in the forced swim and tail suspension tests, and novelty suppressed feeding, measured as a decrease in approach to a novel food item [28, 30, 31]. Mice exposed to CUS also display decreased grooming, aggression, and sexual behaviors. Certain CUS-induced behavioral changes, such as novelty suppressed feeding, can be reversed only by chronic antidepressant treatment [29], making CUS relevant to human antidepressant responses. Female mice display immobility in the forced swim test after just 6 days of subchronic unpredictable stress (SCUS) whereas males are generally resilient to SCUS and require 20-28 days of CUS exposure to elicit depression- and anxiety-like behavior (Hodes, G.E. et al. Soc. Neurosci. Abstr. 219.01, 2011). Interestingly, age is a factor in response to CUS—male rats exposed to 60 days of CUS in the juvenile period exhibit greater memory retention in a two-way shuttle avoidance task compared to rats exposed to the same stressor in adulthood, indicating enhanced cognitive resilience [26]. Sex differences and age effects in susceptibility to CUS-induced depression and anxiety-like behavior make this a powerful tool for investigating the hormonal and neural basis for stress vulnerability and resilience across the lifespan.
2.3 Chronic Social Defeat Stress
Chronic Social Defeat Stress (CSDS) is a prolonged social stress paradigm used to delineate stress resilient and susceptible phenotypes in adult, male rodents. In the CSDS model, a C57BL/6J mouse is repeatedly subordinated by a larger, aggressive CD-1 mouse [32] for 10 consecutive days. Each physical bout is followed by overnight sensory contact with the aggressor through a plastic partition. Following CSDS, approximately 2/3 of experimental mice, termed “susceptible,” develop a constellation of depression-like behaviors including social avoidance and anhedonia [33, 34] as well as metabolic syndrome marked by dysregulated feeding peptides, weight gain and insulin insensitivity [32, 35, 36]. Conversely, the remaining 1/3 of mice, termed “resilient,” develop a much milder phenotype, including elevated corticosterone and increased anxiety-like behavior [33]. Similar to human depression, CSDS-induced depression- and anxiety-like behavior can be reversed by chronic, but not acute, administration of antidepressants [37, 38]. Importantly, a number of biomarkers identified in humans with MDD are similarly disrupted in susceptible mice following CSDS, further highlighting its utility in studying depression mechanisms [33, 39, 40].
2.4 Learned Helplessness
The Learned Helplessness (LH) model is an acute stress paradigm that, similar to CSDS, produces heterogeneous responses, enabling researchers to delineate stress susceptible and resilient animals [28]. The proportion of animals exposed to the LH paradigm that demonstrate phenotypic resilience ranges from 10-80% [41]. In this model, rodents are exposed to repeated inescapable foot shocks followed by a test period in which an easy escape mechanism is made available during shock exposure. Compared to control animals trained with escapable shocks and resilient animals, susceptible animals demonstrate “helplessness,” measured as longer escape latency or failure to escape [42]. Like CSDS, the LH paradigm produces numerous behavioral and physiological changes including weight loss, HPA axis dysfunction, circadian alterations, and reductions in hippocampal synaptic spine number [28]. A weakness of the model is that LH-induced changes are short-lived, usually lasting only 2-3 days and can be reversed with acute antidepressant treatment [41].
3.0 Neuroendocrine Mechanisms of Resilience
Appropriate response to stress involves the coordinated activity of the autonomic nervous system (ANS) and the HPA axis as well as the neural circuits in the hypothalamus, brainstem and forebrain that control their activity (for a comprehensive review, see [43]). Briefly, stress induces ANS processes by first activating preganglionic sympathetic neurons in the spinal cord that project to pre- or paravertebral ganglionic neurons. These neurons terminate on cardiovascular and visceral organs or on the adrenal medulla, and stimulate the release of adrenaline from the adrenal medulla and noradrenaline from sympathetic nerve fibers. Consequences of ANS activation by stress include changes in heart rate and vasoconstriction. In the HPA axis, stress activates neurons in the paraventricular nucleus (PVN) of the hypothalamus to secrete corticotropin releasing factor (CRF) and arginine vasopressin (AVP) into the portal circulation via the median eminence, which in turn stimulate the anterior pituitary gland to release adrenocorticotropic hormone (ACTH). ACTH activates glucocorticoid synthesis and release from the adrenal cortex, which functions primarily to mobilize energy stores during stress. There is ample cross-talk between the ANS and the HPA axis—the adrenal cortex receives innervation from the sympathetic nervous system, regulating glucocorticoid release, and glucocorticoids mediate ANS-dependent stress responses including vasoconstriction. Modulation of these systems has been noted in cases of human resilience to MDD and post-traumatic stress disorder (PTSD), although results have been largely correlative [12]. High dose glucocorticoid administration following traumatic stress exposure has emerged as a potential treatment for individuals vulnerable to PTSD, perhaps working by controlling hyperactive fear response and fear memory consolidation [44]. This strategy has yielded positive results in critically ill hospital patients and combat-exposed veterans [45, 46]. Additionally, dehydroepiandrosterone (DHEA) and neuropeptide Y (NPY) have emerged as potential pro-resilience biomarkers in humans. DHEA is released from the adrenal cortex with cortisol in response to stress and can counter the effects of glucocorticoids [47]. In combat exposed veterans, both DHEA level and DHEA/cortisol ratio correlate negatively with PTSD symptom severity, suggesting that DHEA may serve a protective role in situations of extreme stress. NPY is co-released with noradrenaline from sympathetic nerves and has been shown to correlate positively with interrogation performance and negatively with dissociative symptoms in soldiers undergoing a U.S. Army survival training course [48].
The Hypothalamic Pituitary Gonadal (HPG) axis shares numerous component structures and neural circuitry with the HPA axis, and accordingly, reproductive hormones serve a prominent role in susceptibility and resilience to stress. Mood disorders including MDD and anxiety are about two times more prevalent in adult women than men, a sex difference that emerges in puberty and persists until menopause, suggesting a role for sex hormone fluctuations and activating effects of gonadal hormones on neural circuitry [49-51]. Estrogen supports cognitive processes, influences catecholamine and monoamine neurotransmission and metabolism, and regulates the expression of transcription factors and neurotrophins [52]. Activation of brain stress response and reward circuitry depends on menstrual cycle stage in healthy adult women [53, 54]. Women with a history of MDD display hypoactivation of brain stress response circuitry associated with lower serum estradiol levels and higher serum progesterone levels compared to healthy controls [55]. Mechanistically, perimenopause-associated estradiol fluctuations have been shown to contribute to vulnerability in part by increasing brain levels of monoamine oxidase A (MAO-A), an enzyme involved in apoptosis, oxidative stress, and monoamine metabolism [56]. Conversely, testosterone has emerged as a potential proresilience factor in men [12]. There is a strong positive correlation between testosterone and degree of social connectedness, feelings of personal success, and social dominance [57]. Given its role in social behavior and positive mood, it is not surprising that blood and saliva testosterone levels decrease following stress [58] and that low circulating levels are often found in individuals with PTSD or MDD [59, 60]. Early studies in men suggest that testosterone may be effective in alleviating treatment resistant depression and as an adjunct to treatment with selective serotonin reuptake inhibitors [60]. Although much future work is needed, together this work suggests that testosterone may serve as a pro-resilience factor by promoting positive mood and social connectedness.
3.1 The HPA Axis
Animal studies investigating the mechanistic underpinnings of resilience related to the HPA axis largely focus on models of developmental stress. Adult rats that have undergone stress inoculation in the form of postnatal handling display lower basal levels of CRF, blunted stress-induced increases in ACTH, CRF and corticosterone secretion, and a more rapid post-stress recovery to basal stress hormone levels compared to unstressed rats or those that have undergone maternal separation [20]. Meaney and colleagues [61] have identified maternal care behavior as a mediator of early life stress resilience that produces long lasting individual differences in gene expression and subsequent neuroendocrine stress response. In a study by Liu et al. [62], they report that mothers of handled rats displayed more licking, grooming and arched back nursing behaviors than mothers of nonhandled rats. The amount and frequency of these maternal behaviors correlated negatively with stress-induced plasma ACTH and corticosterone in adulthood [62]. This pro-resilient neuroendocrine stress response in adult offspring of high maternal care mothers resulted from increased hippocampal expression of glucocorticoid receptors (GR), causing greater sensitivity to glucocorticoid negative feedback on CRF and AVP. The group has since identified a number of molecular mediators of enhanced GR expression in handled pups such as increased thyroid hormone secretion, serotonin turnover in the hippocampus, and hippocampal expression of NGF-1A, a cAMP-inducible transcription factor that binds exon 17 of the GR promoter [61, 63, 64]. In adult rats, epigenetic mechanisms maintain glucocorticoid receptor sensitivity in resilient animals. The 5’ CpG dinucleotide site of the NGF-1A consensus sequence on GR is always methylated in offspring of low licking and grooming (LG) mothers whereas it is associated with acetylated H3 in the offspring of high LG mothers [61]. Methylation of this site prevents the binding of NGF-1A to the GR promoter whereas acetylation has the opposite effect. In sum, high LG maternal care produces sustained epigenetic modifications that induce enhanced glucocorticoid receptor expression, enhanced sensitivity to glucocorticoid negative feedback, reduced hypothalamic release of AVP and CRF, and ultimately attenuated HPA axis response to subsequent stress [65].
Although less is known about the HPA mechanisms underlying resilience to adulthood stress, two recent studies identify pro-resilience epigenetic modifications at the CRF gene in PVN neurons and CRF gating of BDNF in the Nucleus Accumbens (NAc) as important mediators. Following CSDS exposure, Elliot et al. [66] reported increased CRF mRNA expression in the PVN and decreased methylation at four CpG sites in the CRF promoter in susceptible, but not resilient, mice. Viral-mediated knockdown of CRF in the PVN after social defeat promoted resilient behavior in the social interaction test, suggesting that CRF promoter methylation in resilient animals underlies adaptive neuroendocrine and behavioral responses. Walsh et al. [67] found that optogenetic induction of phasic firing in dopaminergic neurons of the VTA promoted social avoidance behavior in mice following subthreshold social defeat stress, an effect dependent upon CRF-gated induction of BDNF in the NAc, a structure in which VTA dopaminergic projections terminate. As CRF antagonist infusion blocked the effects of phasic stimulation on social avoidance behavior, CRF is likely an essential mediator of vulnerability and resilience to defeat stress. Future investigation of individual differences in CRF in the NAc will further elucidate CRF activity in resilient animals.
3.2 Gonadal Sex Hormones
The effects of sex hormones on resilience and vulnerability to stress are highly complicated and dependent upon the timing of stress (adulthood vs. developmental) and behavioral domain (cognitive vs. emotional resilience) (See Table 1). Adult female animals tend to show greater cognitive resilience to chronic stress than males, while males display enhanced emotional resilience. Following chronic restraint stress, male rats exhibit deficits in hippocampal-dependent memory tasks including the radial arm maze, object recognition test, Y maze, Morris water maze and object location task, whereas female rats are either unaffected or perform better on spatial and visual memory tasks [68-70]. Mechanisms underlying these differences in cognitive resilience include sex differences in stress-induced changes to hippocampal morphology and corticosteroid receptor sensitivity. Chronic restraint stress induces atrophy of apical dendrites, measured as reduced dendritic length and branch points, in the CA3 region of the hippocampus in male, but not female, rats [71]. Following 21 days of restraint stress, Kitraki et al. [70] reported reduced glucocorticoid receptor (GR) immunoreactivity in the male rat hippocampus and impaired spatial learning and memory in the Morris water maze. In contrast, hippocampal GR and mineralocorticoid receptor immunoreactivity were enhanced in stressed females.
Table 1.
Sexual Dimorphism in Rodent Behavioral Response to Stress
| Stress Paradigm | Age | Duration of Stress | Male Behavioral Phenotype | Female Behavioral Phenotype |
|---|---|---|---|---|
| Paternal Stress | Sires were exposed to stress one month prior to breeding [129] | 10 days of CSDS [129] | • Social avoidance [129] • Anxiety-like behavior [129] • Increased locomotion in response to novelty [129] • Decreased sucrose preference [129] • Increased immobility in the Forced Swim Test (FST) [129] |
• Anxiety-like behavior [129] • Increased immobility in the FST [129] |
| Gestational Stress | Dams were exposed to the stressor during early gestation (days 1-7) [74, 75, 130] | 7 days of chronic variable stress [74, 75, 130] | • Impaired memory acquisition, enhanced recall in the Barnes maze [130] • Increased immobility in the Tail Suspension Test (TST) and FST [74] • Decreased sucrose preference [74] • Locomotor hyperactivity [75] |
• Enhanced memory acquisition in the Barnes maze [130] |
| Early Life Stress | P2-P10 [19] P1-13 [25] P1-14 [131] |
Brief/Mild: 9 days of low corticosterone administration to lactating mother [19], 13 [25] or 14 [131] days of 15-minute maternal separation Prolonged/Severe: 9 days of high corticosterone administration to lactating mother [19], 13 days of 4 hour maternal separation [25], 14 days of 3 hour maternal separation [131] |
Brief/mild: • Enhanced attentional set-shifting performance (cognitive flexibility) [19] • Reduced latency to approach food and water in a novel environment [25, 131] • Decreased startle response [131] • Reduced anxiety-like behavior in the open field [131] Prolonged/severe: • Similar in behavior to control animals [25, 131] |
• The effects of early life stress are poorly understood in females, although recent evidence suggests differential effects of early life stress on exploratory behavior, social behavior and anhedonia [132] |
| Chronic Restraint Stress | 80 days old [70] 2-3 months old [68] |
21 days of 6 hour restraint [68-70] | • Spatial and visual memory deficits (Y maze, Morris water maze, radial arm maze, object recognition, object location) [68-70] | • Normal or enhanced spatial and visual memory [68-70] |
| Chronic Social Defeat Stress | 7-8 weeks old [32-34] | 10 days of 10-minute defeat bouts [32-34] | • “Susceptible” animals display social avoidance, increased anxiety-like behavior and decreased sucrose preference (anhedonia) [33, 34] • “Resilient” animals display increased anxiety-like behavior [33] |
• The CSDS model has not been adequately adapted for use in females due to a lack of conspecific aggressive behavior |
| Unpredictable Stress | Subchronic & Chronic: 7-8 weeks old [27, 133] |
Subchronic: 6 days of varied 1 hour stressors [27, 133] Chronic: 21-28 days of varied 1 hour stressors [133] |
Chronic: • Decreased sucrose preference [133] • Decreased grooming behavior [133] • Increased latency to approach food in a novel environment [133] • Increased immobility in the FST [133] |
Subchronic & Chronic: • Decreased sucrose preference [133] • Decreased grooming behavior [133] • Increased latency to approach food in a novel environment [133] • Increased immobility in the FST [27, 133] |
Our laboratory has identified nuclear factor κB (NFκB) signaling as a potential mediator of emotional resilience to CUS [27]. NFκB is a transcription factor that, although most commonly associated with immune function, can also be activated by stress [72]. The activation and nuclear translocation of NFκB is regulated by the IκB kinase complex (IκK), which triggers the degradation of the cytoplasmic inhibitor of NFκB, IκB [73]. As mentioned earlier, gonadally intact females display depression-like behavior after SCUS, whereas males do not exhibit emotional dysregulation at this time point. Ovariectomy (OVX) abolished this enhanced behavioral susceptibility and blunted transcriptional response to stress—170 genes were regulated by SCUS in OVX mice vs. 619 in gonadally intact females, and many overlapping genes were regulated in opposite directions. Viral overexpression of IκK (significantly upregulated in OVX mice) prevented SCUS-induced immobility in the forced swim test in gonadally intact female mice, whereas overexpression of a dominant negative form of IκK increased immobility in OVX mice. These findings suggest that IκK-NFκB signaling is necessary and sufficient for the expression of resilience following SCUS in females. Additional findings suggest a role for DNA methyltransferase 3a (Dnmt3a) in enhanced male emotional resilience to SCUS. RNA sequencing analysis revealed that female mice exhibit higher expression of Dnmt3a mRNA than do males at baseline. Genetic deletion of Dnmt3a shifts female transcriptional profiles following SCUS exposure toward a more male-like pattern and promotes behavioral resilience to SCUS, whereas viral overexpression of Dnmt3a promotes vulnerability to SCUS. We consistently find that the SCUS-induced transcriptional profiles of male and female mice overlap minimally (Hodes, G.E. et al. Soc. Neurosci. Abstr. 219.01, 2011; Pfau, M.L. et al. Soc. Neurosci. Abstr. 541.26, 2013). Further mining of these data sets may reveal promising patterns and candidate genes for further understanding of sex-dependent stress resilience.
In addition to the activating effects of sex hormones on stress circuitry in adulthood, prenatal perturbations can exert organizational effects on the brain that dictate sex differences in adult stress response. Mueller and Bale [74] reported increased depression-like behavior in male, but not female, mice whose mothers had been exposed to CUS during early pregnancy. Male mice displayed elevated amygdala CRF expression and decreased hippocampal GR expression that corresponded with epigenetic alterations—reduced methylation of the CRF promoter and enhanced methylation of the 17 exon of the GR promoter. The authors identified sex differences in prenatal stress-induced placental gene expression profiles, particularly differences in the methylation maintenance enzyme Dnmt1, as potential developmental mechanisms underlying adult phenotypes. Moreover, a recent study showed that stress-induced pro-inflammatory placental gene expression contributes to enhanced male susceptibility to prenatal stress [75]. Maternal nonsteroidal anti-inflammatory drug treatment reversed the stress-induced increase in placental Interleukin 6 (IL-6) expression and ameliorated locomotor hyperactivity (a behavioral indicator of dopaminergic dysfunction) in prenatally stressed adult male mice. While much work has focused on the maternal environment, an interesting study by Rodgers et al. [76] demonstrated a role for paternal stress in male offspring susceptibility. Adult male mice sired by fathers exposed to CUS in puberty or adulthood displayed HPA axis hypoactivity, which correlated with changes in paternal sperm microRNA expression profiles. Together these results highlight the complex interactions between genetics and environment in stress resilience.
4.0 Immunity & Resilience
The interaction of stress and the immune system has become a major focus of psychiatric research since the introduction of the “cytokine hypothesis of depression” in the 1990s [77]. The hypothesis asserts that many of the central abnormalities observed in depression—enhanced HPA axis activity, neurodegeneration, decreased neurogenesis, oxidative stress, and serotonergic signaling dysfunction—are at least in part due to peripheral inflammatory cytokines released in response to external, psychological stressors and internal stressors such as chronic disease and “leaky gut.”
A growing literature explores the connection between stress, proinflammatory cytokines, and depression and anxiety-like behavior in both humans and animals. Cytokines are soluble proteins that are released at a site of infection by leukocytes. Upon release, they can readily cross the blood brain barrier and act within the central nervous system to mediate sickness behaviors. Sickness behaviors due to inflammation, such as social withdrawal and disinterest in food, overlap greatly with depression behaviors but are attenuated when infection is cleared [78]. Altered regulation of this adaptive behavioral response to immune challenge by chronic illness or psychosocial stress contributes to depression [77, 78]. For example, patients with chronic inflammatory diseases such as multiple sclerosis, rheumatoid arthritis and asthma can be up to 6 times more likely to develop depression than healthy individuals [11]. Depressed patients also show markers of inflammation, including elevated levels of cytokines and their soluble receptors in serum and cerebrospinal fluid, the most consistently elevated being Interleukin-6 (IL-6) [79, 80]. Inflammatory markers are also elevated in rodent stress models—chronic stress causes an elevation in serum and brain cytokines including IL-6 and Interleukin-1β [81-83]. In both humans receiving immunotherapy and animal models of inflammation, administration of pro-inflammatory cytokines produces depression and anxiety-like behaviors [84-87]. While some studies have shown that antidepressant medications reduce peripheral inflammation [88, 89], others suggest the opposite [90, 91], resulting in a shift in drug development efforts that focus on the use of more direct anti-inflammatory agents to promote resilience.
Recent studies form a growing body of evidence that supports the existence of individual differences in inflammatory response to stress and subsequent physiological and behavioral vulnerability. Here, we will discuss peripheral markers characteristic of vulnerability and resilience to stress as well as central mechanisms that contribute to inflammation-mediated behavioral outcomes.
4.1 Heightened Peripheral Inflammation as a Predictor and Consequence of Stress Vulnerability and Resilience
Several reports examine changes in immune cell localization and reactivity driven by stress exposure in rodents. Many of these studies utilize a social stress model similar to CSDS called Social Disruption Stress (SDR). SDR involves chronic disruption of established social hierarchies in cages of male mice. Male cagemates establish a social hierarchy such that one mouse is the dominant, alpha male and the remaining males are codominant or subordinate [92]. Once a day for a total of six days, a novel, dominant intruder mouse previously screened for aggressive behavior is placed into the housing cage for a period ranging from hours to overnight [93]. The dominant intruder repeatedly attacks and defeats the resident mice, eliciting submissive behaviors. SDR has been shown to activate the HPA axis, enhance proinflammatory cytokine release, drive an increase in splenic and blood CD11b+ monocytes via influx from the bone marrow and stimulate glucocorticoid resistance in immune cells [92, 94].
Following SDR, splenic leukocytes from stressed mice release more Tumor Necrosis Factor α (TNF-α) and IL-6 in response to stimulation with lipopolysaccharide (LPS), a bacterial endotoxin and toll-like receptor 4 agonist, compared to leukocytes from control mice, an effect that is driven both by increased number of leukocytes as well as enhanced release from each leukocyte [95]. Enhanced cytokine release likely stems from the glucocorticoid resistance demonstrated by splenic macrophages and monocytes post-SDR, and indicates dysregulation of negative feedback mechanisms by which glucocorticoids and cytokines together self regulate stress-induced hyperinflammation [96]. SDR-induced glucocorticoid resistance in macrophages is at least partly due to a cytokine-mediated failure of corticosterone to stimulate nuclear translocation of glucocorticoid receptors and prevent NFκB-induced proinflammatory transcription [97]. Splenic macrophage enrichment and glucocorticoid resistance is dependent upon Interleukin-1 (IL-1)—mice lacking IL-1 receptor type 1 do not display these phenotypes [94]. Interestingly, Avitsur, Stark and Sheridan [93] observed individual differences in macrophage glucocorticoid resistance based upon level of social subordination. Submissive mice were more likely to develop splenocyte corticosterone insensitivity following SDR than were control or dominant mice. Glucocorticoid resistance correlated negatively with time spent in social exploration and positively with time spent in submissive postures. Level of social exploration prior to SDR exposure was predictive of submissive behavior during the first session of SDR, suggesting that pre-existing differences in mouse behavior may predict response to SDR. Collectively, these results imply that the adaptive mechanism by which corticosterone represses the immune system in response to stress is compromised in susceptible (submissive) mice but maintained in resilient (dominant) mice. Further study is required to determine whether active molecular and cellular mechanisms maintain glucocorticoid sensitivity in resilient mice following SDR exposure and, similar to subordinate behavior, whether baseline differences in these mechanisms can predict ultimate behavioral response. As glucocorticoid resistance is a hallmark symptom of depression, further understanding of immune cell resilience to glucocorticoid insensitivity may prove particularly advantageous for therapeutics.
Recent findings by Hodes et al. (Hodes, G.E. et al. Soc. Neurosci. Abstr. 542.10, 2013) suggest that pre-existing differences in IL-6 signaling from leukocytes also predict behavioral response to CSDS. Prior to any stress, leukocytes from peripheral blood of mice that ultimately became susceptible to CSDS released more IL-6 in response to in vitro immune challenge with LPS. Pro-susceptible mice had higher numbers of circulating leukocytes, and leukocyte number and IL-6 release correlated negatively with social interaction ratio, indicating a predictive relationship. Increased leukocyte number is likely driven by an increase in blood CD11b+ monocytes, as significant differences in proportions of leukocyte subtypes between susceptible and resilient mice were observed only in monocytes. Generation of chimeric mice via transplantation of bone marrow hematopoietic progenitor cells from a susceptible donor produced a robust social avoidance phenotype compared to control bone marrow chimeras. In contrast, chimeras generated via transplantation of progenitors from an IL-6−/− donor demonstrated behavioral resilience to CSDS, behaving similarly to IL-6−/− mice and mice treated with an IL-6 antibody, which binds and neutralizes IL-6 in the peripheral circulation. These findings suggest that peripherally derived IL-6 drives susceptibility to CSDS, and that susceptible and resilient mice display baseline differences in leukocyte number and responsiveness. The mechanisms contributing to pre-existing differences in stress responsive IL-6 release and circulating leukocyte number are under investigation and will inform our understanding of immune regulation in resilience. Experiments investigating whether peripheral blood leukocytes of mice susceptible to CSDS, like splenic leukocytes in mice exposed to SDR, display glucocorticoid resistance may prove particularly fruitful.
As mentioned above, increased levels of CD11b+ monocytes in blood and spleen are both a risk factor for susceptibility to CSDS and a consequence of RSD in mice. A likely mechanism underlying this increased number of monocytes/macrophages is direct sympathetic nervous system innervation of bone marrow and control of bone marrow hematopoiesis via β-adrenergic signaling. Two recent studies propose that stress promotes proliferation and egress of immature, pro-inflammatory myeloid cells from the bone marrow. Powell et al. [98] reported that in mice subjected to RSD, stress induces a transcriptional pattern that ultimately leads to myelopoeisis favoring immature, proinflammatory monocytes and granulocytes that express high and intermediate levels, respectively, of the surface marker Ly6c. RSD results in a 4-fold higher prevalence of monocytes in blood and spleen as well as a 50-70% increase in monocytic and granulocytic bone marrow progenitor cells. Post-RSD genome-wide analysis of the peripheral blood mononuclear cell transcriptome revealed a transcriptional mechanism underlying this phenomenon—enhanced expression of proinflammatory genes and genes related to myeloid cell lineage commitment accompanied by decreased expression of genes related to terminal myeloid differentiation. This transcriptional pattern was dependent upon βadrenergic signaling as administration of propranolol, a β-adrenergic antagonist, prevented pro-inflammatory transcription in peripheral blood monocytes as well as the RSD-induced increase in Ly6chigh monocytes. A transcriptional profile favoring pro-inflammatory monocytes and β-adrenergic signaling was also identified in human subjects of low socioeconomic status, a form of chronic social stress. Further, Heidt et al. [99] found that chronic variable stress increases numbers of monocytes and neutrophils in mouse blood and bone marrow due to proliferation of leukocyte progenitors. Stress-enhanced hematopoietic activity was accompanied by increased bone marrow noradrenaline levels and decreased expression of CXCL12, a negative regulator of hematopoietic stem and progenitor cell (HSPC) proliferation and migration that is in turn regulated by the β3-adrenergic receptor. Treatment of stressed mice with a β3-adrenergic receptor antagonist increased CXCL12 expression, reduced HSPC proliferation and attenuated the stress-induced increase in circulating neutrophils and Ly6chigh monocytes. Together, these studies provide compelling evidence in both humans and mice linking stress vulnerability to sympathetic nervous system mediated leukocytosis. Potentially informative future studies include an investigation of leukocyte population shifts and transcriptional profiles in blood and bone marrow of stress resilient subjects.
Many of the peripheral findings we've discussed focus primarily on stress susceptible animals and suggest immune mechanisms of passive resilience—resilient and control animals lack peripheral markers that are present and detrimental in susceptible animals. However, as research in the field shifts to focus more on pre-existing individual differences in inflammation as a proxy for vulnerability and resilience to depression and anxiety, we anticipate elucidation of active immune mechanisms of resilience, an exciting prospect due to the relative feasibility of therapeutically targeting peripheral systems with monoclonal antibodies, thus reducing off-target effects in the central nervous system.
4.2 Central Immune Regulation of Physiology and Behavior
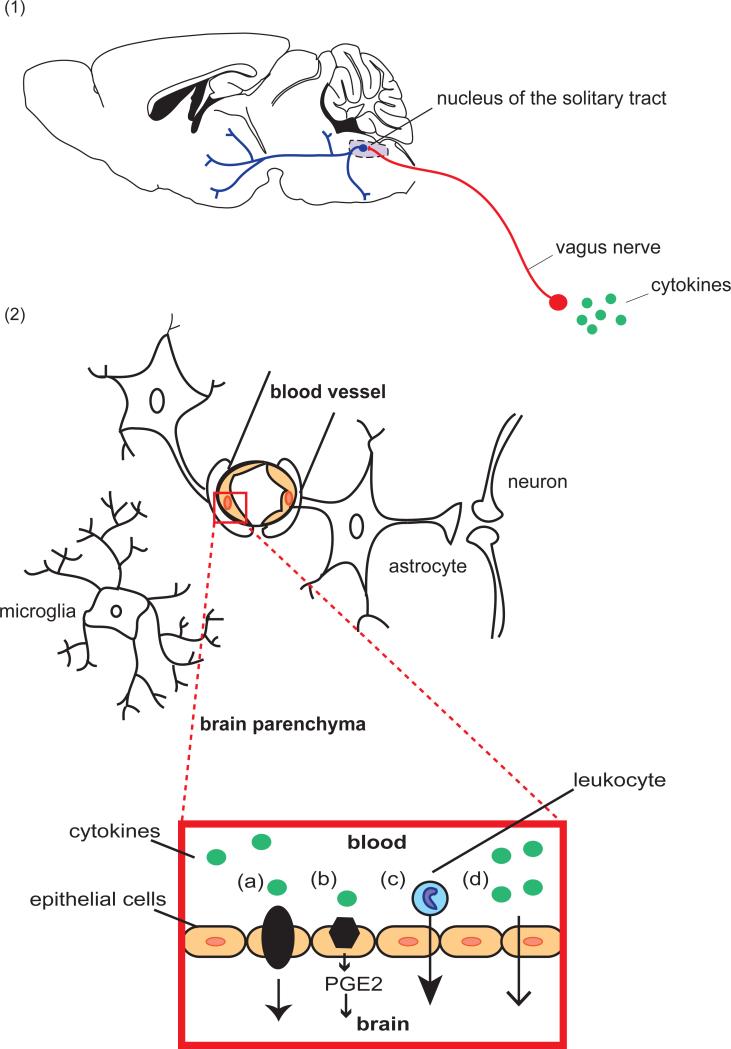
Peripheral cytokine signals reach the central nervous system via two main pathways—stimulation of the vagal nerves and brainstem nuclei (the neural pathway) and crossing of the blood-brain barrier (the humoral pathway, see Figure 1) [78, 100-102]. Centrally derived cytokine signals are produced by microglia, resident brain macrophages. Within the brain, inflammatory signals can influence behavior through mechanisms including activation of the HPA axis and glucocorticoid-induced neuronal atrophy [103] as well as excitatory synaptic plasticity (see Figure 2) [72, 104].
Figure 1. The Interface of the Immune and Central Nervous Systems.
Peripheral immune cells and signals reach the CNS via two primary routes: the neural pathway and the humoral pathway. (1) In the neural pathway, peripheral cytokines activate primary afferent nerves, including the vagus nerve, which terminates in the nucleus of the solitary tract (NTS) in the medulla oblongata. Second order neurons project to brainstem, hypothalamic and forebrain nuclei involved in behavioral stress response. (2) In the humoral pathway, cytokine signals cross the blood brain barrier by transport, diffusion, or trafficking of immune cells. Cytokines in the peripheral circulation cross via cytokine transporters (a) or by activation of cytokine receptors on endothelial cells of the brain vasculature (b), leading to the production of prostaglandins E2 (PGE2), lipophilic molecules that can easily diffuse throughout the brain. Chronic social stress can also promote the recruitment of peripherally derived macrophages into the brain parenchyma of reward regions including the amygdala and prefrontal cortex (c). Cytokines produced locally by macrophage-like cells in the circumventricular organs and choroid plexus cross the BBB by volume diffusion (d). Once within the CNS, peripheral signals activate microglia that in turn produce pro-inflammatory cytokines.
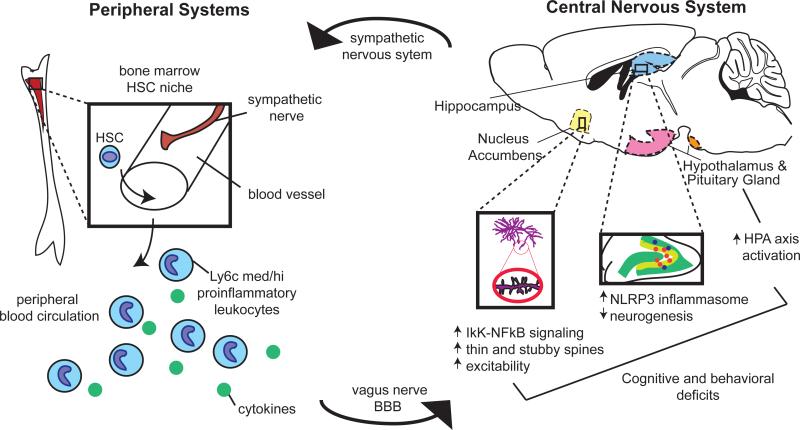
Figure 2. Overview of Immune Activation in the Peripheral and Central Nervous Systems by Stress.
Chronic stress activates the sympathetic nervous system, precipitating a series of immune events in peripheral systems. Sympathetic nerves release noradrenaline in the bone marrow hematopoietic stem cell (HSC) niche, stimulating myelopoeisis favoring the release of immature, proinflammatory monocytes and neutrophils into the peripheral circulation. Cytokine signals derived from these leukocytes and, in some cases, the cells themselves, traffic to the brain by activating afferent nerves or by crossing the blood brain barrier. Within the CNS, they produce lasting changes relevant to depression and anxiety-like behavior by activating the HPA axis, the microglial NLRP3 inflammasome in the hippocampus, and NFκB-IκK signaling in the ventral striatum.
Numerous studies investigating central stress-induced inflammatory processes have revealed a prominent role for Interleukin-1 Beta (IL-1β). Iwata and colleagues [103] propose that stress induces IL-1β release in the brain primarily via the microglial NLRP3 inflammasome. NLRP3 is a cytosolic pattern recognition receptor (PRR) that, when stimulated by toll-like receptor 4 (TLR4) activation or ATP, both of which are regulated by stress, binds to pro-caspase-1, forming the inflammasome complex. Pro-caspase-1 is cleaved and in turn cleaves pro-IL-1β into IL-1β, which is then released from the cell. Microglia constitutively express the components of the NLRP3 inflammasome, and acute restraint stress activates the NLRP3 inflammasome in the hippocampus, the brain region containing the highest concentration of microglia and IL-1β receptors [103, 105]. Intracerebroventricular (i.c.v) administration of IL-1 results in increased anxiety-like behavior in the elevated plus maze and open field as well as spatial memory deficits in the Morris water maze [106]. In contrast, pharmacologic or genetic inhibition of Interleukin-1 Receptor 1 (IL-1R1) blocks anhedonia in rats exposed to CUS [83]. Interestingly, i.c.v administration of an IL-1R1 antagonist prevented shuttle box escape failure following pretreatment with repeated, inescapable tail shocks [107]. These results suggest that IL-1β signaling is an important mediator of behavioral vulnerability and resilience to LH and CUS in rats, and that IL-1β and its downstream effectors may be promising targets for promoting behavioral resilience to stress.
Downstream mechanisms by which IL-1β influences behavioral outcomes to stress include HPA axis activation as well as modulation of hippocampal neurogenesis. Stress-induced IL-1β modulates the HPA axis by stimulating release of CRF from the hypothalamus and subsequent downstream release of ACTH from the pituitary gland [103, 108, 109]. Blockade of IL-1R1 via antagonist administration or null mutation prevents CUS-induced reductions in cells positive for BrdU (Bromodeoxyuridine, a marker of cell division) and DCX (doublecortin, a marker of immature neurons), indicating that chronic stress inhibits neurogenesis in an IL-1β dependent fashion [83]. In the same study, in vitro incubation with IL-1β decreased the proliferation of adult hippocampal progenitor cells, an effect blocked by co-incubation with inhibitors of NFκB signaling. As the IκK-NFκB signaling pathway is activated by IL-1β and other pro-inflammatory cytokines, it is a promising candidate mediator of the downstream effects of IL-1β. A follow-up study revealed that, indeed, exposure to an acute stressor activated NFκB signaling in neural stem-like cells (NSCs), and NFκB activation in NSCs was dependent upon IL-1β signaling [110]. Moreover, i.c.v. administration of an NFκB inhibitor throughout CUS blocked the subsequent stress-induced decrease in BrdU+DCX+ cells as well as the expression of anxiety-like and anhedonic behaviors.
Our lab has reported IκK-NFκB dependent structural and functional plasticity in the mesolimbic dopaminergic reward pathway following CSDS [72]. IκK and its downstream targets IκB and phosphorylated IκB were upregulated in the nucleus accumbens (NAc) of susceptible mice following CSDS. Interestingly, activation of IκK-NFκB signaling promotes susceptibility to CSDS by altering plasticity of glutamatergic synapses in the NAc. Strategies to blunt IκKNFκB activation directly in NAc promote resilience. A subsequent study revealed that constitutive viral overexpression of IκK promotes baseline anxiety and depression-like behaviors in the open field and forced swim tests as well as social avoidance and anhedonic behavior in response to an acute social defeat stress [111]. IκK expression induced the formation of immature spines (primarily thin spines) in mice exposed to acute social defeat stress. Again, spine density correlated significantly with social avoidance behavior, suggesting that IκK-dependent, stress-induced morphological changes may drive behavioral response to stress. Together, these data suggest a critical role for IκK-NFκB signaling in NAc in susceptibility versus resilience to social stress. Future studies will be important to identify the upstream inflammatory signaling pathways responsible for such effects.
5.0 Molecular and Cellular Mechanisms of Resilience in the Central Nervous System
Much of our current knowledge regarding central mechanisms of resilience centers on mesocorticolimbic reward circuitry. Brain reward circuitry serves the adaptive purpose of focusing one's attention on the acquisition of natural rewards to ultimately ensure survival [112]. Mesocorticolimbic circuitry comprises neurons from the medial prefrontal cortex (mPFC), hippocampus, nucleus accumbens (NAc), amygdala, ventral tegmental area (VTA), lateral hypothalamus, and lateral habenula, among other brain regions (see Figure 3). Collectively, these brain regions are involved in numerous psychological and cognitive processes that are impacted by stress and compromised in patients with depression or anxiety [113]. Connections between mesocorticolimbic regions are dense and often complex. Here, we will focus primarily on the most well characterized connections, those of the VTA-NAc reward circuit. Dopaminergic neurons of the VTA project onto GABAergic medium spiny neurons (MSNs) of the NAc, a structure within the ventral striatum. VTA neurons release dopamine in response to reward-related stimuli to initiate consumption and sometimes also in response to aversive stimuli. The NAc sends reciprocal connections back to the VTA via two pathways—the direct pathway, via D1-type MSNs; and the indirect pathway, via D2-type MSNs, which innervate GABAergic interneurons in the ventral pallidum that in turn synapse onto VTA neurons. Although findings are variable, human imaging studies support a role for the VTA-NAc pathway in human depression, demonstrating consistent reductions in NAc activity in adult patients as well as NAc volume reductions in elderly patient populations [114-117]. Deep brain stimulation procedures targeting the NAc and its efferent connections in the VTA have shown good therapeutic efficacy in treatment resistant depression (T. Schlaepfer, personal communication). However, it is currently unknown how these stimulation protocols affect NAc microcircuitry and whether they indirectly stimulate fibers of passages that synapse outside the NAc.
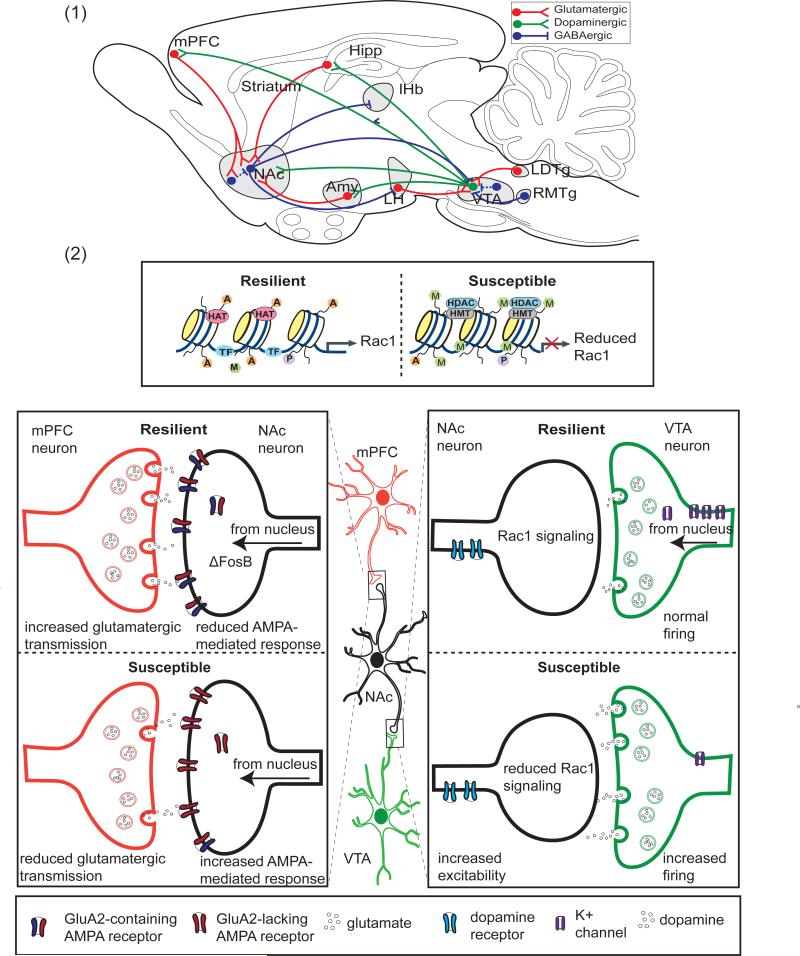
Figure 3. Molecular and Cellular Mechanisms of Resilience in the Mesocorticolimbic Reward Circuitry.
(1) A simplified schematic of the VTA-NAc circuit, showing the major glutamatergic, GABAergic, and dopaminergic projections to and from the Ventral Tegmental Area (VTA) and Nucleus Accumbens (NAc) in the rodent. Dashed lines indicate internal, inhibitory connections mediated by interneurons. Hipp, Hippocampus; mPFC, Medial Prefrontal Cortex; Amy, Amygdala; LH, Lateral Habenula, RTMg, rostromedial tegmentum; LDTg, Lateral Dorsal Tegmentum. (2) Shown are examples of processes that contribute to resilience vs. susceptibility to chronic social defeat stress. Within the NAc, resilient mice lack the reduced Rac1 gene transcription characteristic of susceptible animals and attributed to reduced histone pan-acetylation (specified by A) and enhanced lysine 27 methylation (specified by M). In susceptible animals, reduced expression of Rac1 leads to actin cytoskeletal reorganization, and an increase in stubby, immature spines. Also in NAc neurons, ΔFosB activity promotes resilience by increasing expression of GluA2. Although shown here for simplicity in the synapse, ΔFosB is a transcription factor, and its activity occurs in the nucleus. GluA2-containing AMPA glutamate receptors are Ca2+ impermeable, reducing AMPA-mediated glutamate response and excitability. In the mPFC, increased glutamatergic transmission has been associated with resilience, and optogenetic stimulation of ChR2 in the mPFC has been shown to promote resilience. In the VTA, resilient animals lack the stress-induced increase in dopaminergic phasic firing that drives susceptibility. Increased firing in susceptible animals is driven by an increased cationic current. Resilient animals homeostatically maintain normal firing rate through the induction of K+ channels. HAT, histone acetyltransferase; HDAC, histone deacetylase; HMT, histone methyltransferase; P, phosphorylation; TF, transcription factor.
5.1 Epigenetic and Transcriptional Mechanisms
Numerous epigenetic and transcriptional mechanisms in mesocorticolimbic reward circuitry underlie antidepressant action and resilient behavioral responses to chronic stress. The transcription factor ΔFosB is upregulated in the NAc of resilient mice following CSDS in a serum response factor (SRF) dependent manner, and genetic overexpression or antagonism of ΔFosB expression promotes behavioral resilience or susceptibility, respectively [118, 119]. Furthermore, ΔFosB levels are reduced in postmortem NAc samples of human depressed patients. Chronic fluoxetine treatment enhances ΔFosB concentration in the mouse NAc, and ΔFosB is required for fluoxetine-mediated antidepressant effects in susceptible mice. ΔFosB exerts its pro-resiliency effects through its transcriptional targets, including AMPA glutamate receptor subunit GluA2 and Sparc-like 1 (SC1). Following CSDS, resilient mice show greater NAc expression of GluA2 than do control or susceptible mice, an effect mediated by ΔFosB binding to the GluA2 promoter. ΔFosB-mediated enhanced GluA2 expression promotes resilience by decreasing AMPA function—GluA2-containing AMPA receptors are Ca2+ impermeable with lower receptor conductance and reduced inwardly rectifying currents. In addition, SC1, a protein localized to the PSD and necessary for proper synapse assembly, is upregulated both in mice overexpressing ΔFosB and in mice resilient to CSDS. SC1 overexpression reverses social avoidance behavior following CSDS.
Epigenetic regulation of ras-related C3 botulinum toxin substrate 1 (Rac1) has been shown by our laboratory to mediate susceptibility versus resilience to CSDS [39]. Rac1 is a Rho GTPase involved in the organization and maintenance of the actin cytoskeleton, largely through regulation of its downstream target cofilin, an actin severing protein critically involved in synaptic plasticity. Following CSDS, Rac1 was downregulated in the NAc of susceptible, but not resilient, mice, and its expression correlated with social avoidance behavior. Viral-mediated overexpression and knockdown experiments demonstrated that Rac1 is necessary and sufficient for the expression of resilient behavior following CSDS. ChIP analysis of permissive H3 acetylation (acH3) and repressive histone H3 Lys9 trimethylation (H3K9me3) at the Rac1 promoter and its upstream regions revealed that, in both susceptible and resilient mice, Rac1 expression was mediated by epigenetic mechanisms. Susceptible, but not resilient, mice exhibited reduced permissive acetylation at the Rac1 promoter and its 2,000-bp upstream region. Resilient mice showed reduced methylation within the Rac1 promoter whereas susceptible mice showed enhanced methylation in the 1,000-bp upstream region. Chronic intra-NAc administration of an HDAC inhibitor reversed social avoidance behavior and rescued Rac1 expression in susceptible mice. Collectively, these results suggest that epigenetic mechanisms maintain Rac1 expression in resilient mice, promoting adaptive behavioral response to stress, but have the opposite effect in susceptible mice. Analysis of human postmortem NAc tissue samples from depressed patients corroborated these animal findings. Rac1 expression was strongly reduced in unmedicated patients compared to controls, and depressed patients showed decreased acetylation in regions ~200-bp upstream and downstream of the transcription start site (TSS) accompanied by increased methylation in the gene region ~200-bp upstream of the TSS. Rac1 likely promotes resilient responses to CSDS via its effects on MSN spine structure. Viral-mediated overexpression of Rac1 reduced the CSDS-induced enhancement in dendritic stubby (immature) spine density whereas Cre-mediated Rac1 genetic deletion has the opposite effect.
5.2 Neurophysiology of Susceptibility and Resilience in the VTA
A robust neurophysiological correlate of susceptibility to CSDS is the enhanced excitability of VTA dopamine neurons following stress [120, 121]. CSDS increases the spontaneous firing rate of VTA dopamine neurons and the percentage of neurons demonstrating burst firing events in susceptible, but not resilient, mice [121]. These physiological changes correlate inversely with social interaction score and can be reversed with chronic antidepressant treatment, suggesting an involvement of stress-induced changes to neuronal excitability in depression-like behavior. One mechanism underlying enhanced excitability in susceptible mice is the Ih (hyperpolarization-activated cation) current [121]. The Ih current regulates tonic firing of dopamine neurons as well as the transition from single-spike to burst firing, and is robustly increased only in susceptible mice following CSDS. Ih inhibitor infusion reverses social avoidance behavior, and chronic antidepressant treatment reduces the stress-induced increase in Ih current. Enhanced neuronal excitability is also mediated by reduced activation of AKT (thymoma-viral proto-oncogene) in the VTA, which likely produces a subsequent reduction in inhibitory tone [120]. Phosphorylated AKT is reduced in the VTA of susceptible mice following CSDS, and this reduction is necessary and sufficient to produce social avoidance behavior. AKT targets the β2 subunit of the GABAA receptor, and in vitro pharmacological reduction of AKT reduces the amplitude and frequency of spontaneous GABA-mediated inhibitory postsynaptic currents (IPSCs) onto VTA dopaminergic neurons. Future in vivo studies are needed to causally link AKT-GABA changes to social avoidance behavior. Recently, Chaudhury, Walsh and colleagues [122] demonstrated that the CSDS-induced high frequency phasic firing in dopamine neurons of the VTA-NAc pathway is sufficient to functionally drive susceptible behavior. Optogenetic induction of phasic, but not tonic, firing in tyrosine hydroxylase positive (TH+) VTA neurons during or after exposure to subthreshold defeat rapidly produced robust social avoidance and anhedonia behaviors. Induction of phasic firing during the social interaction test following 10 days of CSDS was sufficient to reverse behavior in mice previously identified as resilient, generating social avoidance, and to produce long-lasting changes in excitability, as evidenced by maintenance of depression-like behavior (decreased sucrose preference) 8-12 hours post-stimulation. These effects were VTA-NAc pathway specific, as selective optogenetic stimulation of VTA TH+ neurons projecting to the PFC did not induce social avoidance or anhedonia. Halorhodopsin inhibition of VTA firing reversed depression-like behavior in susceptible mice following CSDS exposure. These experiments demonstrate that stress-induced phasic firing in NAc-projecting VTA dopamine neurons is necessary and sufficient for the development of depression-like behavior.
Normal dopamine neuron firing rate, AKT activation and signaling, and Ih current dynamics are allostatically preserved in resilient mice during and after stress exposure, although the mechanisms underlying this allostasis are less understood than those driving susceptibility. A recent study by Friedman et al. [123] identified an active mechanism by which normal dopamine neuron firing is maintained in resilient mice. Surprisingly, VTA dopamine neurons of resilient animals do not show a return to a normal Ih current comparable to that of controls following CSDS. Instead, they exhibit an Ih current increase that is much larger than that of susceptible mice. Underlying this phenomenon is a homeostatic enhancement in multiple K+ channel currents—the potentiated Ih current augments neuronal firing to such an extent that K+ channels are activated, returning firing rate to a normal level. Indeed, current injection in dopamine neurons of resilient mice produces a reduction in spike number, whereas current injection produces the opposite effect in susceptible mice. Repeated intra-VTA infusion of lamotrigine, an Ih potentiator, or VTA viral-mediated overexpression of hyperpolarization-activated and cyclic nucleotide-gated channel 2 (HCN2), a channel that regulates Ih current, reversed social avoidance and anhedonic behavior in susceptible mice. Both manipulations increased Ih and K+ currents, and reduced neuronal excitability. Further, repeated optogenetic induction of hyperactivity in VTA dopamine neurons increased K+ currents and reversed social avoidance behavior. This type of homeostatic plasticity was specific to VTA-NAc projecting neurons. These findings provide exciting insight into the biology of resilience as well as a potential therapeutic avenue.
5.3 Glutamatergic Synapses in Resilience and Reward
In addition to dopaminergic innervation from the VTA, the NAc also receives glutamatergic innervation from the PFC, amygdala, thalamus and hippocampus. Decreased PFC activity, as measured by cerebral blood flow and glucose metabolism, is the most robust finding reported by human imaging studies of depressed patients [124]. Findings from rodent models are generally consistent with those in humans and suggest that stress leads to hypofrontal function. First, chronic stress leads to significant atrophy and synapse loss on glutmatergic neurons in the PFC [113, 125, 126]. Importantly, loss of synapses has also been observed in the PFC of humans with MDD [127]. Covington et al. [128] reported decreased expression of the immediate early genes (IEGs) zif268 (also termed egr1) and arc in human postmortem prefrontal cortical tissue of unmedicated depressed patients. IEG expression was also reduced in the ventromedial PFC of susceptible mice, but was unchanged in resilient mice following CSDS. As IEG expression is considered a representation of brain activity, these results suggest that activity is reduced in susceptible mice and depressed patients, but maintained in resilient mice. Optogenetic stimulation of the mPFC of susceptible mice had an antidepressant effect, reversing social avoidance and anhedonic behavior, and indicating that burst firing in mPFC neurons promotes behavioral resilience. Optogenetic induction of burst firing also increased expression of the IEG c-fos. The NAc is another region of brain reward circuitry that undergoes significant stress-induced remodeling of glutamatergic synapses. Following CSDS, susceptible, but not resilient, mice have an increased density of glutamatergic synapses on NAc MSNs, which correlates with increased mini excitatory postsynaptic potential (mEPSP) frequency (indicative of more functional glutamatergic synapses or altered presynaptic release). Data from our lab using circuit specific optogenetic tools to stimulate glutamatergic neurons terminating in the ventral striatum (vStr), find that glutamatergic projections from the intralaminar thalamus (ILT) promote susceptibility to CSDS whereas stimulation of projections from the PFC exert opposite effects (Christoffel, D.J. et al. Soc. Neurosci. Abstr. 705.08, 2013). Both chronic, viral-mediated expression in the ILT of tethered toxins (tToxins, designed to inhibit excitatory transmission by selectively blocking calcium influx at the pre-synaptic voltage gated Ca2+ channels Cav2.1 and Cav2.2) and rapid optogenetic inhibition of ILT-vStr terminal projections prevented social avoidance and reduced MSN stubby spine density (a parameter that is known to positively correlate with social avoidance). Accordingly, rapid terminal stimulation of ILT projections to the vStr was pro-susceptible, inducing social avoidance in a subthreshold defeat. Together, these findings suggest that ILT-vStr projections are necessary and sufficient for the expression of social avoidance. In line with previous work on the role of PFC activity in depression-like behavior, chronic tToxin-mediated inhibition of PFC projections was pro-susceptible. Surprisingly, the more specific, rapid optogenetic inhibition of PFC-vStr glutamatergic terminals failed to induce social avoidance. This indicates that PFC-mediated resilience may require sustained activation of PFC-NAc terminals or the activity of other PFC terminal projections outside the striatum. While the PFC may provide a promising target for promoting resilience to stress, further research is needed to fully elucidate (1) the particular anatomical and physiological parameters of pro-resilient PFC activity, and (2) whether allostatic mechanisms maintain normal PFC-vStr firing patterns in resilient animals to prevent pathological changes in reward circuit activity.
6.0 Conclusion
With the exception of the rapidly acting antidepressant ketamine and the advent of deep brain stimulation paradigms to treat depression, both of which are limited to severe, treatment resistant cases of depression, there has been a decades long void of new treatment options for depression and anxiety. However, the future of treatment and research is hardly dire. Modern research on stress-related disorders has yielded numerous potential targets and biomarkers for diagnosis and treatment, largely due to an enhanced focus on alternatives to monoamine-based mechanisms, such as epigenetic mechanisms, immune-related factors, sex, and the biology of resilience. Stress-related disorders, and resilience to them, can be considered products of the coordinated activity of the brain and numerous bodily systems. The results of resilience research we’ve described here are particularly exciting as they offer an opportunity for personalized science and medicine. We’ve described potential targets and biomarkers specific to type of stress (developmental vs. adulthood), sex, and inflammatory state. As women are more likely to suffer from mood disorders, the continuing identification of sex-based, pro-resilience markers may enable the development of more effective, sex specific treatments. The NIH-mandated inclusion of female subjects in research studies will hopefully encourage further elucidation of sex-based resilience. We feel that immune mechanisms are particularly promising as many potential targets are peripheral, removing the blood-brain barrier as a therapeutic obstacle. Preclinical experiments in our lab indicate that peripherally targeting IL-6 with monoclonal antibodies is antidepressant in mice (Hodes, G.E. et al. Soc. Neurosci. Abstr. 542.10, 2013). Further research on active mechanisms that maintain normal immune function in resilient individuals, particularly the maintenance of glucocorticoid sensitivity and normal leukocyte differentiation and release from bone marrow, may yield additional therapeutic options for peripheral administration. An enhanced focus throughout the field on individual differences in response to stress and inclusion of resilient animals as research subjects is necessary, particularly in regard to studies of the immune system, where study of stress-resilient subjects has been minimal. Further interrogation of the mechanisms of what we’ve termed “passive” resilience will also be helpful. As described in this review, the adaptive failure of resilient animals to display the pathological markers seen in susceptible animals is often accomplished by active mechanisms. An enhanced focus on resilient subjects may enable us to harness mechanisms of resilience in the body and brain for the successful treatment of stress-related disorders.
Acknowledgements
This research was supported by US National Institute of Mental Health grants R01 MH090264 (SJR), R01 MH104559 (SJR), R21 MH099562 (SJR), Janssen/IMHRO Rising Star Award (SJR), F31 MH105217 (MLP), T32 MH087004 (MLP) and T32 MH096678 (MLP).
Abbreviations
- CUS
chronic unpredictable stress
- SCUS
subchronic unpredictable stress
- CSDS
chronic social defeat stress
- LH
learned helplessness
- LG
licking and grooming
- OVX
ovariectomy
- SDR
social disruption stress
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masten AS. Ordinary magic. Resilience processes in development. The American psychologist. 2001;56:227–38. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 2.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature reviews. Neuroscience. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–3. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Alim TN, Feder A, Graves RE, Wang Y, Weaver J, Westphal M, et al. Trauma, resilience, and recovery in a high-risk African-American population. Am J Psychiatry. 2008;165:1566–75. doi: 10.1176/appi.ajp.2008.07121939. [DOI] [PubMed] [Google Scholar]
- 5.Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of personality and social psychology. 2003;84:365–76. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? The American psychologist. 2004;59:20–8. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of aging. 2002;23:921–39. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 12.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nature neuroscience. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter M. Implications of resilience concepts for scientific understanding. Annals of the New York Academy of Sciences. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- 14.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews. Neuroscience. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 15.Macri S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neuroscience and biobehavioral reviews. 2011;35:1534–43. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: Implications for understanding resilience. Developmental psychobiology. 2010;52:402–10. doi: 10.1002/dev.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–55. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Archives of general psychiatry. 2004;61:933–41. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 19.Macri S, Granstrem O, Shumilina M, Antunes Gomes dos Santos FJ, Berry A, Saso L, et al. Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Hormones and behavior. 2009;56:391–8. doi: 10.1016/j.yhbeh.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research. Molecular brain research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 21.Macri S, Wurbel H. Effects of variation in postnatal maternal environment on maternal behaviour and fear and stress responses in rats. An Behav. 2007;73:171–84. [Google Scholar]
- 22.Levine S. Plasma-Free Corticosteroid Response to Electric Shock in Rats Stimulated in Infancy. Science. 1962;135:795–&. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- 23.Levine S, Haltmeye Gc, Karas GG, Dennenbe Vh. Physiological and Behavioral Effects of Infantile Stimulation. Physiol Behav. 1967;2:55–&. [Google Scholar]
- 24.Young LD, Suomi SS, Harlow HF, McKinney WT., Jr. Early stress and later response to separation in rhesus monkeys. Am J Psychiatry. 1973;130:400–5. doi: 10.1176/ajp.130.4.400. [DOI] [PubMed] [Google Scholar]
- 25.Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–24. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 2012;15:11–20. doi: 10.3109/10253890.2011.572207. [DOI] [PubMed] [Google Scholar]
- 27.LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–80. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Current topics in behavioral neurosciences. 2011;7:121–47. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 30.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behavioural brain research. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Feng SF, Shi TY, Fan Y, Wang WN, Chen YC, Tan QR. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behavioural brain research. 2012;232:245–51. doi: 10.1016/j.bbr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr. Effects of Striatal DeltaFosB Overexpression and Ketamine on Social Defeat Stress-Induced Anhedonia in Mice. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaouloff F. Social stress models in depression research: what do they tell us? Cell and tissue research. 2013;354:179–90. doi: 10.1007/s00441-013-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lazaro E, Arregi A, Beitia G, Vegas O, Azpiroz A, Garmendia L. Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress. 2011;14:537–48. doi: 10.3109/10253890.2011.562939. [DOI] [PubMed] [Google Scholar]
- 37.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 38.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 39.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nature medicine. 2013;19:337–44. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robison AJ, Vialou V, Sun HS, Labonte B, S AG, Dias C, et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39:1178–86. doi: 10.1038/npp.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 42.Seligman MEP, Beagley G. Learned Helplessness in Rat. J Comp Physiol Psych. 1975;88:534–41. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews. Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: a review. Depression and anxiety. 2012;29:833–42. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, D DEQ, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Annals of the New York Academy of Sciences. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 46.Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2010;22:274–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta psychiatrica Scandinavica. 2006;114:187–93. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 48.Morgan CA, 3rd, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–9. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 49.Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33:3–17. doi: 10.1016/j.psyneuen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Holden C. Sex and the suffering brain. Science. 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- 51.Epperson CN, Kim DR, Bale TL. Estradiol Modulation of Monoamine Metabolism: One Possible Mechanism Underlying Sex Differences in Risk for Depression and Dementia. JAMA psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Human brain mapping. 2014;35:847–65. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–70. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, et al. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131:379–87. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rekkas PV, Wilson AA, Lee VW, Yogalingam P, Sacher J, Rusjan P, et al. Greater Monoamine Oxidase A Binding in Perimenopausal Age as Measured With Carbon 11-Labeled Harmine Positron Emission Tomography. JAMA psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards DA, Wetzel K, Wyner DR. Intercollegiate soccer: saliva cortisol and testosterone are elevated during competition, and testosterone is related to status and social connectedness with team mates. Physiol Behav. 2006;87:135–43. doi: 10.1016/j.physbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Morgan CA, 3rd, Wang S, Mason J, Southwick SM, Fox P, Hazlett G, et al. Hormone profiles in humans experiencing military survival training. Biol Psychiatry. 2000;47:891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- 59.Mulchahey JJ, Ekhator NN, Zhang H, Kasckow JW, Baker DG, Geracioti TD., Jr. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001;26:273–85. doi: 10.1016/s0306-4530(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 60.Pope HG, Jr., Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:105–11. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- 61.Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–63. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 63.Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, et al. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–35. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 65.Kappeler L, Meaney MJ. Epigenetics and parental effects. BioEssays : news and reviews in molecular, cellular and developmental biology. 2010;32:818–27. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- 66.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nature neuroscience. 2010;13:1351–3. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 67.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nature neuroscience. 2014;17:27–9. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–16. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 69.Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of learning and memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 70.Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Annals of the New York Academy of Sciences. 2004;1018:323–7. doi: 10.1196/annals.1296.039. [DOI] [PubMed] [Google Scholar]
- 71.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 72.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–21. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohamed MR, McFadden G. NFkB inhibitors: strategies from poxviruses. Cell cycle. 2009;8:3125–32. doi: 10.4161/cc.8.19.9683. [DOI] [PubMed] [Google Scholar]
- 74.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–46. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–12. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic brain disease. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 78.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–8. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 80.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 81.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Translational psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013;8:e58488. doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. Journal of clinical psychopharmacology. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 85.Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry research. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 86.Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–56. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 87.Sakic B, Gauldie J, Denburg JA, Szechtman H. Behavioral effects of infection with IL-6 adenovector. Brain Behav Immun. 2001;15:25–42. doi: 10.1006/brbi.1999.0576. [DOI] [PubMed] [Google Scholar]
- 88.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. Journal of clinical psychopharmacology. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Kubera M, Maes M, Holan V, Basta-Kaim A, Roman A, Shani J. Prolonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depression. J Affect Disord. 2001;63:171–8. doi: 10.1016/s0165-0327(00)00182-8. [DOI] [PubMed] [Google Scholar]
- 90.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–9. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immunol Allergy Clin North Am. 2009;29:285–93. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and behavior. 2001;39:247–57. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 94.Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–17. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–7. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;280:R1799–805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 97.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. Journal of neuroimmunology. 2003;137:51–8. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 98.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–9. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nature medicine. 2014 doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nature reviews. Endocrinology. 2012;8:743–54. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Molecular neurobiology. 2008;37:142–52. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–14. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31:5414–25. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–63. [PubMed] [Google Scholar]
- 106.Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- 107.Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain research. 1995;695:279–82. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- 108.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–4. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 109.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–6. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 110.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, et al. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–23. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews. Neuroscience. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Reviews in the neurosciences. 2011;22:535–49. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry research. 1991;40:95–9. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 115.Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Archives of general psychiatry. 1992;49:553–7. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 116.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 118.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nature neuroscience. 2010;13:745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, et al. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J Neurosci. 2010;30:14585–92. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–8. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367:2475–84. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature medicine. 2012;18:1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–90. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–14. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 131.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 132.Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Frontiers in psychiatry. 2013;4:78. doi: 10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hodes GE. Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biology of sex differences. 2013;4:1. doi: 10.1186/2042-6410-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]