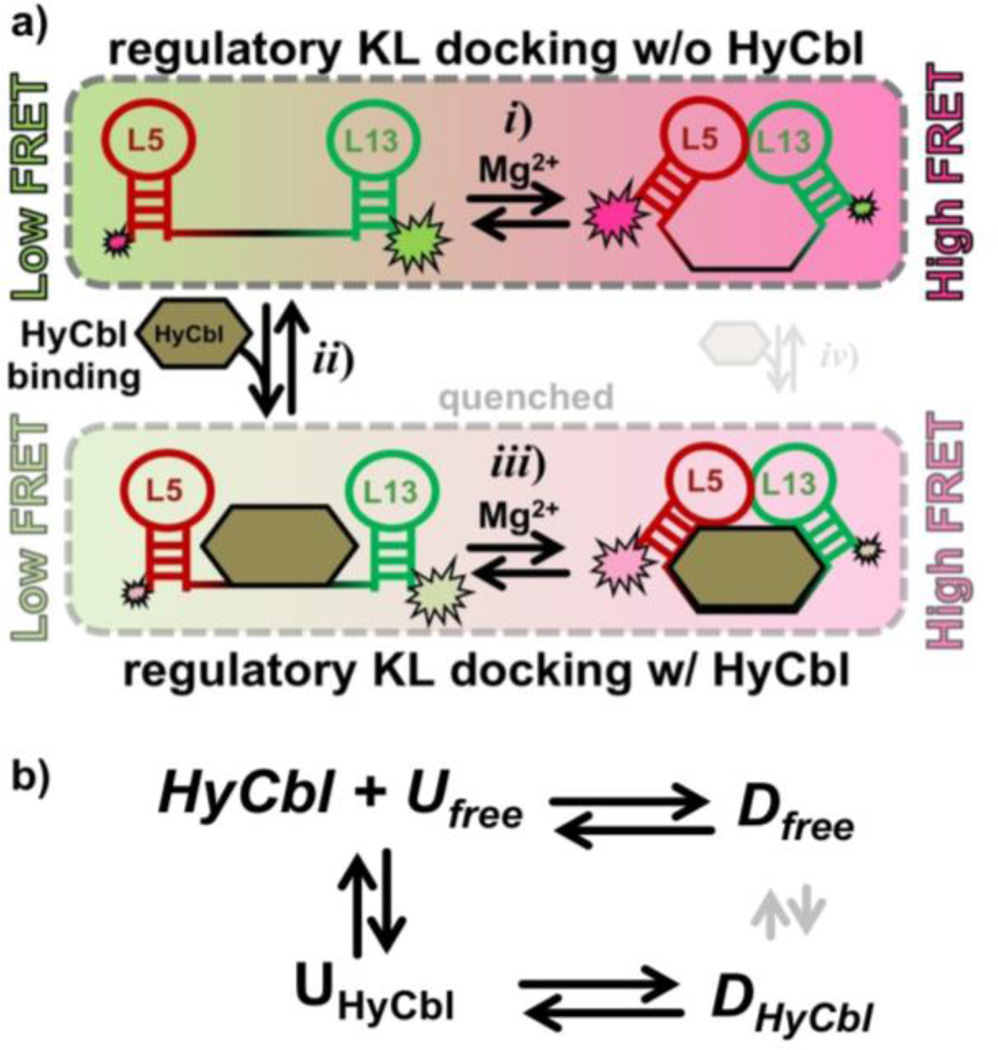
Figure 2.
Four-state kinetic model for the env8HyCbl riboswitch represented a) graphically and b) symbolically. The four macroscopic conformations are linked by three coupled equilibria: (i) KL docking in the absence of ligand, (ii) ligand binding in the undocked conformation, and (iii) KL docking in the presence of ligand. Formation of the KL decreases the inter-dye distance, resulting in more efficient fluorescence resonance energy transfer (EFRET). Ligand binding quenches Cy3, which decreases the total fluorescence of Cy3 and Cy5 and can therefore be monitored independently of KL docking/undocking events. Although it is possible that a fourth (iv) equilibrium exists, we observe no experimental evidence for such a process (Section 3.3), indicating that this process is prevented by prohibitively large free energy barriers (as indicated by the small transparent arrows). Accordingly, this process is not considered for simplicity and ease of discussion; its inclusion would not alter the interpretations and conclusions derived from this work.