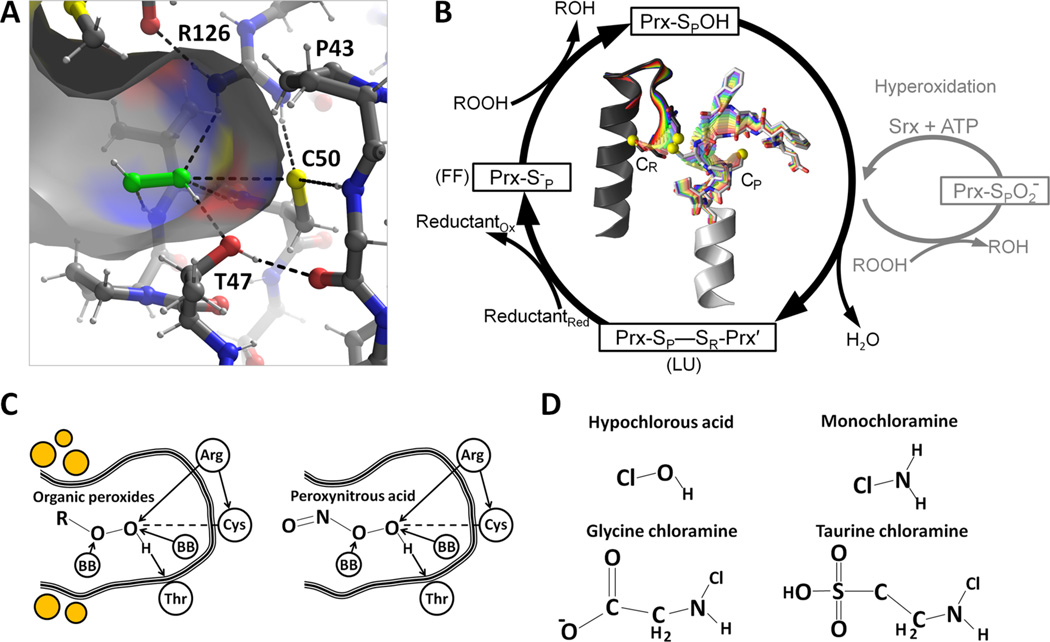
Figure 2.
Catalysis by peroxiredoxins. (A) Michaelis complex of peroxide (green) bound to the FF active site of ApTpx (PDB entry 3a2v) with atom coloring (gray carbons, white hydrogens, yellow sulfurs, red oxygens, and blue nitrogens) showing key hydrogen bonds (dashed lines). (B) The normal Prx catalytic cycle (black) is shown along with the hyperoxidation shunt (gray). To illustrate the change in conformation necessary for Prx catalysis, the center shows a morph between FF and LU conformations for the Prx1 subfamily member StAhpC; the CP- and CR-containing chains are colored white and dark gray, respectively, and the C-terminal region beyond CR is not shown. (C) An organic peroxide and peroxynitrous acid are shown bound to the active site in ways that mimic the interactions made by peroxide in panel A. “BB” refers to a backbone NH hydrogen bond donor. The placement of the hydrophobic collar seen in some organic peroxide selective Prxs is noted by orange circles. (D) Chemical structures of some other molecules recently reported to react with Prxs (see the text).