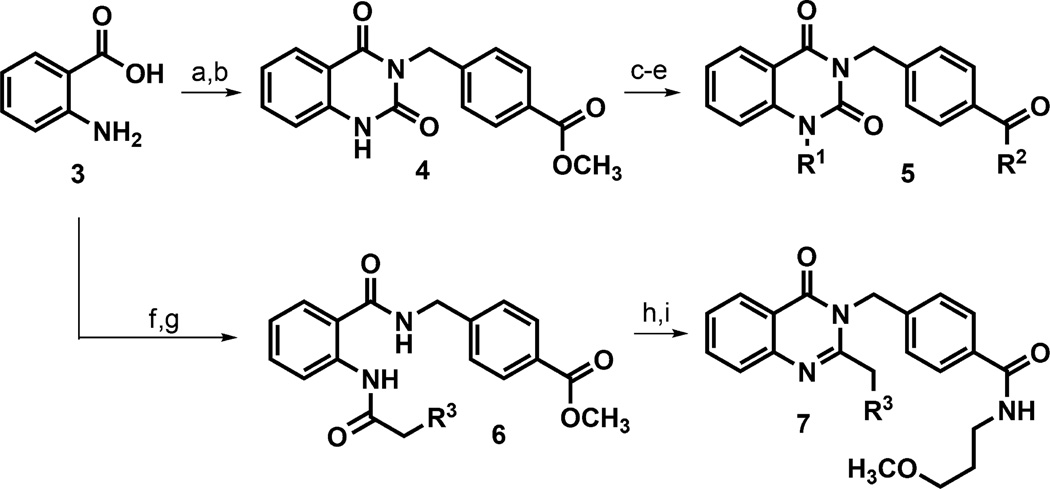
Scheme 1. Chemical Synthesis of Quinazolinedione and Quinazolinone Analogsx.
xReagents: (a) DIPEA, HATU, methyl 4-(aminomethyl)benzoate hydrochloride, DMF, rt, 2 h, 68%; (b) DIPEA, 1,1-carbonyldiimidazole, CH2Cl2, reflux, 16 h, 95%; (c) LiOH·H2O, THF, 40 °C, 1 h, 88%; (d) DIPEA, HATU, 3-methoxypropylamine or other amine for R2, DMF, rt, 2 h, 44–76%; (e) K2CO3, 4-isopropylbenzyl bromide other aryl halide for R1, DMF, 40 °C, 16 h, 11–71%; (f) DIPEA, EDCI, HOBt, methyl 4-(aminomethyl)benzoate hydrochloride, CH2Cl2, rt, 74%; (g) 3- or 4-isopropylphenylacetic acid, (COCl)2, cat. DMF, CH2Cl2, then pyridine, CH2Cl2, 1.5 h, rt, 93–100%; (h) LiOH, THF, H2O, 40 °C, 20 h; (i) 3-methoxypropylamine, EDCI, HOBt, DIPEA, CH2Cl2, rt, 10–22% over 2 steps.