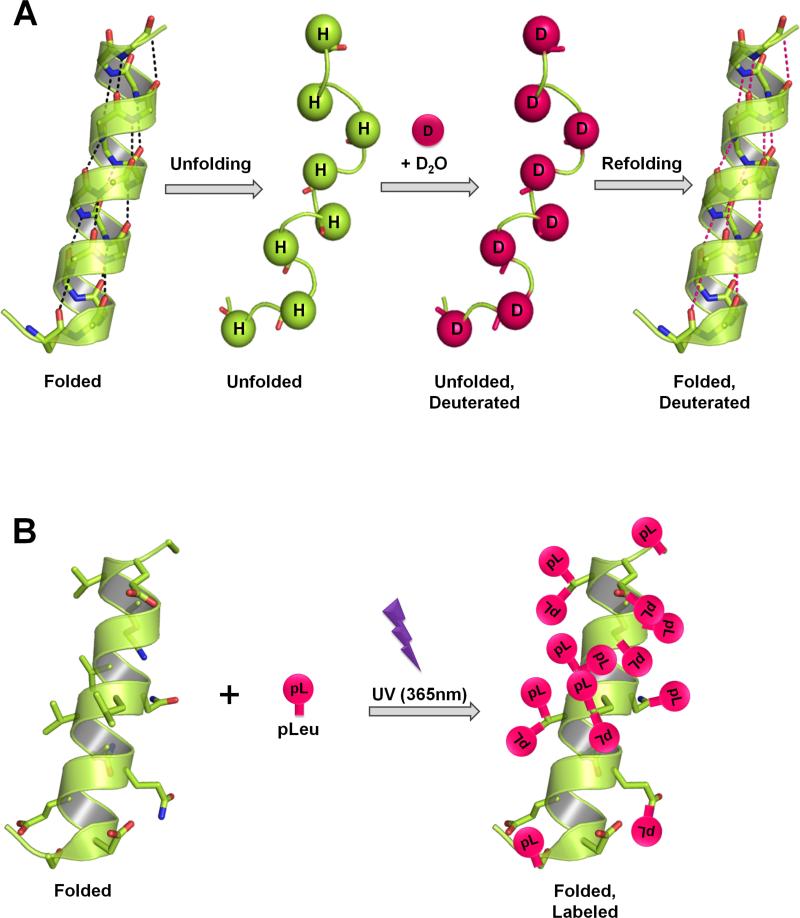
Figure 1. ssHDX and ssPL measure protein structure in lyophilized solids through different labeling mechanisms.
(A) In HDX, the backbone amide hydrogens exchange with deuterium as a function of protein structure and D2O accessibility. In the solid-state, the rate and extent of deuterium exchange depend on the level of D2O sorption, protein mobility (unfolding and refolding events) and the nature of the excipients present in the solid matrix. (B) In PL, UV irradiation at 365 nm initiates the formation of a reactive carbene intermediate from the diazirine functional group of pLeu and is inserted non-specifically into any X-H bond (X= any atom), or added across a C=C bond in its immediate vicinity. In the solid-state, the rate and extent of labeling depend on the local concentration of the labeling agent, irradiation time, protein structure and the nature of excipients present in the solid matrix. Figure A and B show the maximum theoretical labeling that can occur on backbone and side-chains respectively in protein.