Figure 1.
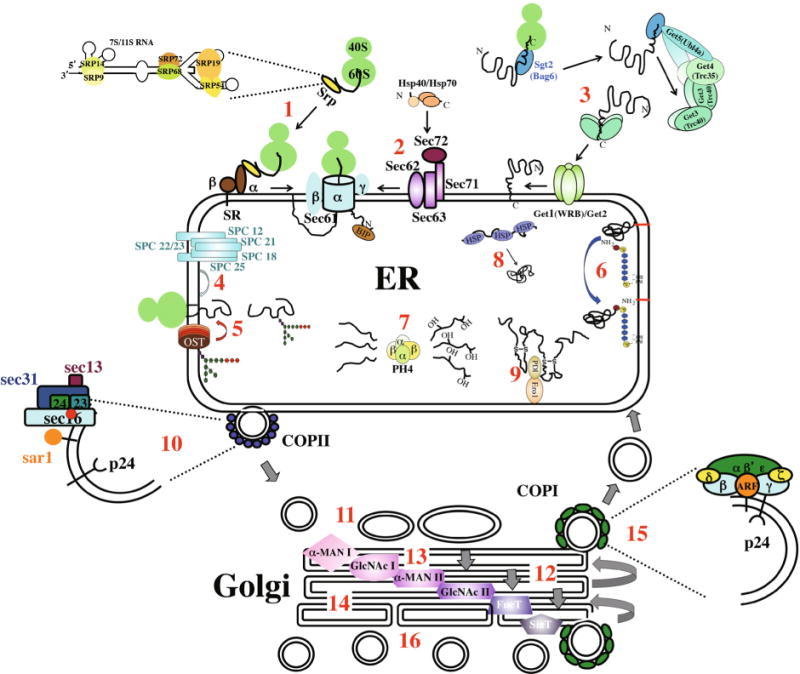
Major components of the secretory system are illustrated, from ER entry (steps 1–3) to post-Golgi sorting of vesicles containing fully processed cargo molecules (step16). Co-translational translocation involves the signal recognition particle (SRP), the signal recognition particle receptor (SR) and the Sec61 translocon complex (Sec61) (1). Post-translational translocation involves cytosolic chaperones (Hsp40s and Hsp70s), the Sec63 complex and the Sec61 translocon (2). Insertion of tail-anchored proteins into the ER membrane requires the Get pathway (3). Removal of N-terminal signal sequences requires the SPase complex (4). N-linked glycosylation requires oligosaccharide transferase (OST) to recognize the consensus sequence and attach the precursor glycan to the protein (5). Both steps 4 and 5 occur as protein is still being synthesized. GPI anchor addition involves cleavage of a C-terminal, membrane-spanning hydrophobic domain and covalent attachment of the lipid moiety (6). The hydroxylation of proline residues in ECM proteins, such as collagens, is mediated by Prolyl-4-hydroxylase (7). Protein folding in the ER is assisted by a large number of chaperone proteins, many of them HSPs (8). Many secreted cargo and surface proteins form disulfide bonds between cysteine residues (9). Anterograde trafficking between the ER and Golgi is mediated by COPII vesicles (10), which dock and fuse to either the cis-Golgi or ERGIC (11). Trafficking through the Golgi is likely to occur by a Golgi maturation mechanism (12), where earlier Golgi compartments are reformed by fusion of retrograde trafficking of COPI vesicles containing compartment-specific enzymes (13). O-linked glycosylation – the addition of N-acetyl-galactosamine sugars to oxygen atoms in polypeptides – also occurs in the Golgi (14 – not illustrated). COPII vesicles are also involved in retrograde trafficking from the Golgi to ER (15). Cargo is sorted for transport to specific final destinations (lysosomes, endosomes, plasma membrane) in the trans-Golgi (16). Some evidence suggests some compartmentalization of the trans-Golgi with respect to the ultimate destinations of cargo proteins.
