Abstract
N-7 and 2′-O methylation of host cell mRNA occurs in the nucleus and results in the generation of cap structures (cap 0, m7GpppN; cap 1, m7GpppNm) that control gene expression by modulating nuclear export, splicing, turnover, and protein synthesis. Remarkably, RNA cap modification also contributes to mammalian cell host defense as viral RNA lacking 2′-O methylation are sensed and inhibited by IFIT1, an interferon (IFN) stimulated gene (ISG). Accordingly, pathogenic viruses that replicate in the cytoplasm have evolved mechanisms to circumvent IFIT1 restriction and facilitate infection of mammalian cells. These include: (a) generating cap 1 structures on their RNA through cap-snatching or virally-encoded 2′-O methyltransferases, (b) using cap-independent means of translation, or (c) using RNA secondary structural motifs to antagonize IFIT1 binding. This review will discuss new insights as to how specific modifications at the 5′-end of viral RNA modulate host pathogen recognition responses to promote infection and disease.
Keywords: interferon, viral pathogenesis, RNA structure, methylation, innate immunity, immune, evasion
Pathogen associated molecular patterns (PAMPs) that are unique to viral RNA (e.g., 5′-ppp-RNA or double-stranded RNA) are recognized by Toll-like receptors (TLR3, TLR7, and TLR8) and the RIG-I-like receptors (RLR: RIG-I and MDA-5) in the host cell endosome and cytoplasm. Upon binding their respective PAMPs these pathogen recognition receptors (PRRs) trigger signaling cascades that induce nuclear translocation of transcription factors (IRF-3, IRF-7, and NF-κB), which induce expression of antiviral type I interferons (IFN-α and -β) (reviewed in (Bowie and Unterholzner, 2008)). IFN-α and -β bind to and signal through the type I IFN receptor (IFNAR) in an autocrine and paracrine manner to induce the expression of hundreds of IFN-stimulated genes (ISGs) that have diverse antiviral and immune regulatory functions. Recent studies have identified an additional PAMP on some viral mRNA lacking-2′-O methylation, which is recognized by the ISG Ifit1 (Habjan et al., 2013; Hyde et al., 2014; Kimura et al., 2013; Menachery et al., 2014; Szretter et al., 2012). As mRNA of higher eukaryotic organisms contain 2′-O methylation on their 5′ cap structures, Ifit1 may have evolved, in part, to distinguish self from non-self RNA.
Eukaryotic N-7- and 2′-O-methylation of host mRNA
RNA methylation is a common post-transcriptional modification that regulates gene expression by influencing diverse aspects of RNA biology including nuclear export, splicing, transcript stability, and translation. In eukaryotic cells, host mRNAs are capped at the 5′ end by an inverted N-7 methyl guanosine nucleoside (m7GpppN), which is linked to the RNA moiety by a triphosphate bridge (Fig 1A). The presence of the m7G cap (cap 0) structure enhances mRNA translation by promoting the association of translation initiation factors such as eIF4E with the 5′ end, as transcripts lacking methylated 5′ caps fail to complex efficiently with 40S ribosomal subunits (Both et al., 1975; Gebauer and Hentze, 2004; Shatkin, 1985). Additionally, methylated 5′ cap structures stabilize RNA transcripts by preventing degradation by 5′-3′ cellular exoribonucleases (reviewed in (Garneau et al., 2007)), and regulate early steps in RNA transcription by associating with gene promoters (reviewed in (Bentley, 2005; Pei et al., 2003; Schroeder et al., 2000)).
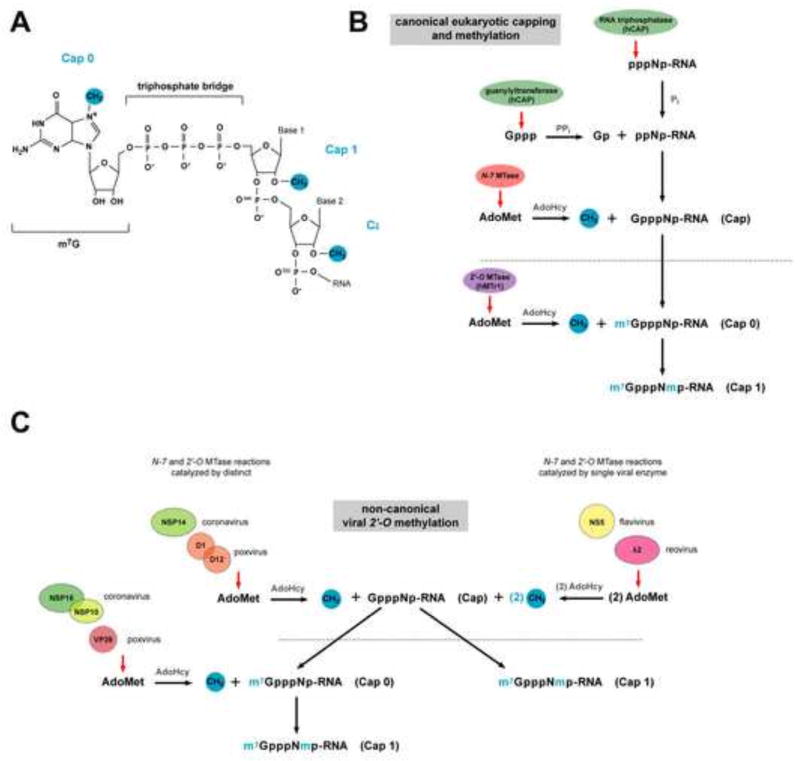
Figure 1. Overview of cap 1 structure formation in eukaryotic and viral systems.
(A) Chemical structure of the G cap and methylation sites which form cap 0, cap 1, and cap 2 structures. (B) The eukaryotic canonical capping pathway. Newly transcribed RNA (pppNp- RNA) is cleaved by RNA triphosphatase at the γ-phosphate to yield diphosphate RNA (ppNp- RNA). Guanylyltransferase, which forms part of the bifunctional capping enzyme (hCAP) that also encodes triphosphatase activity, then hydrolyzes the α-phosphate from GTP and transfers the GMP moiety to the ppNp-RNA acceptor to generate GpppNp. GpppNp-RNA is methylated by the N-7 MTase, which transfers a methyl group from an S-adenosyl-L-methionine (AdoMet) donor to the unmethylated G-cap acceptor, producing cap 0 RNA (m7GpppNp) and S-adenosyl- L-homocysteine (AdoHcy) as a by-product. The 2′-O-ribose MTase (hMTr1) then transfers a methyl group from the AdoMet donor to the first and second nucleotides of cap 0 RNA to generate cap 1 and cap 2 structures, respectively. (C) Generation of viral cap 1 structures through non-canonical capping and methylation pathways. Analogous to eukaryotic RNA methylation, some viruses (e.g. coronavirus and poxvirus) catalyze cap 0 and cap 1 formation separately via two distinct enzymes or enzymes complexes (left). In contrast, other viruses (e.g. flavivirus and reovirus) catalyze N-7 and 2′-O methylation sequentially via a single enzyme (right). Although the cap 1 end product is identical between host and viral transcripts structural differences in the viral and eukaryotic enzymes that catalyze these reactions and their distinct modes of ligand binding make viral 2′-O MTases potential targets for inhibitor design.
Addition of the m7G cap occurs co-transcriptionally in the nucleus via a three-step enzymatic reaction (Fig 1B). Newly transcribed RNA (pppNp-RNA) is cleaved by RNA triphosphatase at the γ-phosphate to yield diphosphate RNA (ppNp-RNA) (Yagi et al., 1983). Guanylyltransferase, which forms part of the bifunctional capping enzyme (hCAP) that also encodes triphosphatase activity, then hydrolyzes the α-phosphate from GTP and transfers the GMP moiety to the ppNp-RNA acceptor to generate GpppNp (Venkatesan et al., 1980; Venkatesan and Moss, 1980; Yagi et al., 1983; Yamada-Okabe et al., 1998). This reaction shows specificity for ppNp-RNA as pppNp-RNA is capped far less efficiently, and pNp-RNA not at all. GpppNp-RNA is then methylated by a distinct enzyme, (guanine-N-7-)methyltransferase (N-7 MTase), which modifies the 5′ terminal guanosine of dinucleoside and polynucleoside triphosphate moieties (Ensinger and Moss, 1976). In this reaction the N-7 MTase transfers a methyl group from an S-adenosyl-L-methionine (AdoMet) donor to the unmethylated G-cap acceptor, producing cap 0 RNA (m7GpppNp) and S-adenosyl-L-homocysteine (AdoHcy) as a by-product.
Whereas N-7-methylation is conserved among all eukaryotes, 2′-O-ribose methylation of the first or the first and second transcribed nucleotides immediately downstream of the m7G cap (which forms cap 1 and cap 2 structures, respectively) is present only in mRNA of bony fish and higher eukaryotes (Varela et al., 2014). The additional 2′-O methylation event and generation of cap 1 structures facilitates splicing of small nuclear RNAs (snRNA) (Donmez et al., 2004) and enhances translation of mRNA during oocyte maturation (Kuge et al., 1998; Kuge and Richter, 1995). Beyond these functions, 2′-O methylation and cap 1 structures contribute to the recognition and restriction of non-self RNA, particularly in the context of the cell-intrinsic immune response to viruses (Daffis et al., 2010; Habjan et al., 2013; Hyde et al., 2014; Kimura et al., 2013). Indeed, expression of the MTase (hMTr1, also known as ISG95) that catalyzes formation of cap 1 structures is augmented by interferon (IFN), supporting a role for differential methylation of RNA cap structures in immune detection and restriction (Haline-Vaz et al., 2008).
2′-O-ribose methylation of host cell mRNA occurs co-transcriptionally in the nucleus through the actions of hMTr1 (Belanger et al., 2010; Pei et al., 2003; Schroeder et al., 2000). Analogous to the nuclear N-7 MTase, hMTr1 associates with Pol II (Haline-Vaz et al., 2008) and can methylate both m7GpppG and GpppG RNA in vitro (Belanger et al., 2010; Langberg and Moss, 1981; Smietanski et al., 2014). The crystal structure of the human 2′-O-MTase catalytic domain complex (Smietanski et al., 2014) has revealed that recognition of the guanosine cap by eukaryotic and viral 2′-O-MTases occurs in a distinct manner (Egloff et al., 2002; Egloff et al., 2007; Hodel et al., 1998). Proposed differences in the structural interaction of host and viral 2′-O-MTases with their RNA ligands has been used as the basis for development of targeted viral MTase inhibitors, as discussed below.
2′-O-methylation and host-mediated restriction of viral RNA
2′-O methylation of cap structures contributes to the sensing of non-self RNA and restriction of viral replication and pathogenesis. Ifit1, an IFN-induced RNA-binding protein, mediates this effect by preferentially binding to viral RNA lacking 2′-O-methylation at their 5′ end and inhibiting RNA translation (Daffis et al., 2010; Kimura et al., 2013; Kumar et al., 2014; Li et al., 2013; Menachery et al., 2014; Szretter et al., 2012; Zhang et al., 2014; Zust et al., 2011; Zust et al., 2013). Introduction of loss-of-function point mutations into the catalytic tetrad (KDKE) of flavivirus, coronavirus, and poxvirus 2′-O-MTases (e.g., West Nile virus NS5-E218A, Japanese encephalitis virus NS5-E218A, Dengue virus NS5-E216A and E217A, mouse hepatitis virus NSP16 D130A, human coronavirus 229E NSP16-D129A, SARS-coronavirus NSP16-D130A, and vaccinia virus J3-K175R) resulted in attenuated virus infections both in vitro and in vivo (Daffis et al., 2010; Kimura et al., 2013; Kumar et al., 2014; Li et al., 2013; Menachery et al., 2014; Szretter et al., 2012; Zhang et al., 2014; Zust et al., 2011; Zust et al., 2013). Mutations that abrogated 2′-O methylation were associated with decreased viral replication in wild-type mice and cells, and an increased sensitivity to the antiviral actions of type I IFN. Replication and virulence was restored in the absence of an intact IFN signaling response or Ifit1 (Daffis et al., 2010; Szretter et al., 2012; Zust et al., 2011) in primary cell culture (dendritic cells, macrophages, and neurons) or in vivo. Attenuated 2′-O-MTase mutant viruses have potential as vaccines as they retain immunogenicity and confer protection against challenge with pathogenic flaviviruses, rhabdoviruses, and coronaviruses (Li et al., 2013; Ma et al., 2014; Menachery et al., 2014; Zhang et al., 2014; Zust et al., 2013).
Ifit1 belongs to a family of IFN-induced proteins (human IFIT1 and IFIT1B, IFIT2, IFIT3, and IFIT5; mouse Ifit1, Ifit1b, Ifit1c, Ifit2, and Ifit3) that are induced rapidly and expressed to high levels in an IFN-dependent and -independent manner following virus infection (Guo et al., 2000b; Kitamura et al., 2001; Sharma et al., 2003; Smith and Herschman, 1996; Terenzi et al., 2005). IFIT genes exhibit low sequence identity between both orthologs and paralogs (31 to 69%) but are related by their multiple tetratricopeptide repeat (TPR) domains (Smith and Herschman, 1996). The TPR motif comprises a degenerate 34 amino-acid sequence containing eight loosely conserved hydrophobic residues, and is important in mediating protein-protein interactions (Goebl and Yanagida, 1991; King et al., 1995; Lamb et al., 1995; Terlecky et al., 1995; Tzamarias and Struhl, 1995). TPR motifs form an amphipathic antiparallel helix and higher order helical structures, although the overall structure may vary considerably among proteins (reviewed in (D’Andrea and Regan, 2003)).
The crystal structures of the N-terminus of human IFIT1, the full-length IFIT2 homodimer, and full-length IFIT5 monomer have helped to elucidate to how IFIT proteins interact with RNA ligands (Abbas et al., 2013; Feng et al., 2013; Katibah et al., 2013; Yang et al., 2012). Although a complete structure of mouse or human Ifit1 has not been determined, the sequence and structural homology between the N-terminal subdomains of IFIT1 and IFIT5 suggest a conserved mode of RNA ligand binding (Abbas et al., 2013). IFIT1, IFIT2, and IFIT5 all form superhelical structures containing a highly positively charged grove or pocket, which accommodates binding of different RNA ligands. Although the precise mode and specificity of RNA binding differs among IFIT proteins (Table 1), mutation of positively charged residues lying within this pocket is sufficient to abrogate RNA binding (Abbas et al., 2013; Katibah et al., 2013; Kumar et al., 2014; Pichlmair et al., 2011). IFIT1 also may also undergo conformational changes upon ligand binding, as has been observed for IFIT5 (Abbas et al., 2013; Katibah et al., 2014). Unbound IFIT5 adopts a relatively open conformation near the binding pocket, presumably to facilitate interaction with different RNA ligands; upon association with RNA, structural changes result in a more closed conformation (Katibah et al., 2014).
Table 1.
Summary of Ifit-ligand binding
| 5′-OH | 5′-ppp | Cap (GpppNp) | Cap 0 (7mGpppNp) | Cap 1 (7mGpppNm) | References | |
|---|---|---|---|---|---|---|
| Human IFIT1 | − | − (>1.4 μM) + (242 nM) |
+ | + (23 ± 4 nM) | − | (Habjan et al., 2013; Pichlmair et al., 2011) (Kumar et al., 2014) (Abbas et al., 2013 |
| Human IFIT1B | + | + | (Kumar et al., 2014) | |||
| Mouse Ifit1 | − | − + |
+ | + | − | (Habjan et al., 2013; Kimura et al., 2013; Pichlmair et al., 2011) |
| Mouse Ifit1c | − | + | − | (Habjan et al., 2013) | ||
| Rabbit IFIT1 | − (>1.4 μM) | + (20 ± 1 nM) | − | (Kumar et al., 2014) | ||
| Rabbit IFIT1B | − | + (9 ± 2 nM) | + (457 ± 24 nM) | (Kumar et al., 2014) | ||
| Human IFIT2 | − + (dsRNA) |
− + (dsRNA) |
− | − | − + |
(Habjan et al., 2013; Kumar et al., 2014; Pichlmair et al., 2011; Yang et al., 2012) |
| Mouse Ifit2 | − | − | − | (Habjan et al., 2013) | ||
| Human IFIT3 | − | − | − | − | − + |
(Abbas et al., 2013; Habjan et al., 2013; Kumar et al., 2014; Pichlmair et al., 2011) |
| Mouse Ifit3 | − | − | − | (Habjan et al., 2013) | ||
| Human IFIT5 | − | + (372 ± 21 nM) (1.4 ± 0.18 μM) |
− | − + (1.7 ± 0.15 μM) |
− | (Abbas et al., 2013; Feng et al., 2013; Habjan et al., 2013; Katibah et al., 2014; Kumar et al., 2014) |
No or minimal binding (−), positive binding (+). Dissociation constant (KD) is indicated in parentheses. Unavailable data is indicated by grey cells. Specificity for dsRNA binding is indicated (dsRNA).
Biochemical analysis also has contributed to our understanding of the specificity and dynamics of Ifit1-RNA binding (Table 1). Initial studies indicated that IFIT1 interacted with RNA transcripts containing 5′-ppp moieties (Pichlmair et al., 2011) in a manner similar to IFIT5 (Abbas et al., 2013; Feng et al., 2013; Katibah et al., 2013; Pichlmair et al., 2011). Surface plasmon resonance, filter binding, primer extension inhibition, and electromobility shift assays, all have defined a low affinity (KD ~250 nM to > 1 μM) interaction between IFIT1 and 5′-ppp RNA (Kumar et al., 2014; Pichlmair et al., 2011), with one study indicating this strength of binding was sufficient to sequester negative strand RNA and inhibit infection of influenza A, vesicular stomatitis, and Rift Valley fever viruses (Pichlmair et al., 2011). IFIT1/Ifit1 binds more avidly (KD ~ 9 to 30 nM) to viral or host cap 0 RNA lacking 2′-O methylation in a sequence-independent manner (Habjan et al., 2013; Hyde et al., 2014; Kimura et al., 2013; Kumar et al., 2014). The binding of cap 0 RNA by IFIT1 appears unique as IFIT2/Ifit2, IFIT3/Ifit3, and IFIT5 fail to bind with appreciable affinity (Habjan et al., 2013; Kumar et al., 2014). Although N-7-methylation of RNA (m7GpppNp-RNA) was not required for IFIT1 binding, this modification enhanced binding to IFIT1 and IFIT1B compared to unmethylated G-capped RNA (GpppNp-RNA). Nucleotides proximal to the methylated cap 0 and triphosphate bridge likely modulate IFIT1-RNA interactions, as primer extension assays suggest that at least ~4 or 5 nucleotides downstream of the cap 0 structure are bound by IFIT1 (Kumar et al., 2014). Analogously, nucleotides proximal to cap structures also enhance binding of other cap-binding proteins to RNA (Chung et al., 1994; Kumar et al., 2014). This concept is supported by recent studies demonstrating that RNA secondary structure can inhibit Ifit1 binding independent of cap methylation status (Hyde et al., 2014), possibly through steric hindrance of Ifit1-RNA interactions. The influence of N-7- methylation status of cap 0 structures on IFIT1 binding may be species-specific, as differences in ligand binding affinities were observed for rabbit IFIT1 (Kumar et al., 2014).
Varying ligand specificities of human IFIT1 and IFIT1B suggests overlapping yet distinct biological functions of IFIT1-like genes. In contrast to IFIT1, which binds cap 0 but not cap 1 RNA, the paralog IFIT1B binds both cap 0 and cap 1 structures (Kumar et al., 2014). This cap 1 binding activity has fostered the hypothesis that IFIT1B has additional translational control functions in the absence of an antiviral response. This is supported indirectly by the observations that IFIT1B expression is not induced by IFN or PAMP stimulation (Fensterl and Sen, 2011; Liu et al., 2013), and can bind non-viral RNA ligands (Kumar et al., 2014).
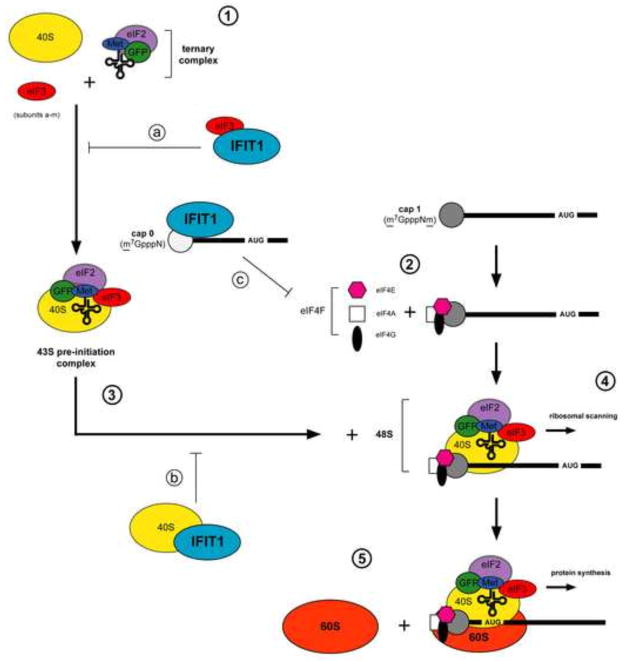
Although IFIT1 has been implicated as an IFN-induced protein that inhibits translation of viral mRNA, the precise mechanism has remained unclear (Andrejeva et al., 2013; Guo et al., 2000a; Habjan et al., 2013; Hui et al., 2003; Hui et al., 2005; Kimura et al., 2013; Kumar et al., 2014; Wang et al., 2003). In canonical cap-dependent translation (Fig 2) (reviewed in (Jackson et al., 2010)), the 40S ribosomal subunit complexes with eIF3 and the ternary complex (eIF2- GTP-Met-tRNA) to form the 43S pre-initiation complex. This pre-initiation complex is recruited to mRNA via cap-binding complex eIF4F (eIF4A, eIF4E, eIF4G), which together forms the 48S complex and scans mRNA sequences for the AUG initiator codon. Earlier studies suggested that Ifit1 and IFIT1 bind to subunits of the eIF3 complex to prevent downstream events required for translation initiation (Guo et al., 2000a; Hui et al., 2003; Hui et al., 2005; Wang et al., 2003). The interaction of IFIT1 with components of the eIF3 complex is species-specific, as human IFIT1 targets eIF3e, which inhibits association with the ternary complex (Hui et al., 2003). More recently, IFIT1 has been suggested to inhibit translation at the step of 48S complex formation via interaction with the 40S subunit (Kumar et al., 2014). In comparison, mouse Ifit1 reportedly interacts with eIF3c to prevent eIF4F-mediated recruitment of the 43S pre-initiation complex to RNA (Hui et al., 2005).
Figure 2. Ifit1 inhibits diverse aspects of RNA translation.
Canonical cap-dependent translation proceeds as follows: (1) the 40S ribosomal subunit complexes with eIF3 and the ternary complex (eIF2-GTP-Met-tRNA) to form the 43S pre-initiation complex. (2) eIF4E associates with the cap and recruits the eIF4F cap-binding complex (eIF4A, eIF4E, eIF4G). (3) eIF4F facilitates unwinding of the RNA and the 43S pre-initiation complex is recruited to form the 48S complex. (4) The 48S complex scans along the mRNA for the AUG initiator codon. (5) The 60S ribosomal subunit is recruited to the 48S complex to form the 80S initiator complex, which translates the mRNA. Ifit1/IFIT1 binds to subunits of the eIF3 complex (a) to prevent ternary complex formation or 43S formation and recruitment (Guo et al., 2000a; Hui et al., 2003; Hui et al., 2005; Kumar et al., 2014; Wang et al., 2003). (b) IFIT1 interaction with the 40S ribosomal subunit abrogates 48S formation (Kumar et al., 2014). (c) Ifit1/IFIT1 directly associates with cap 0 viral RNA and inhibits recruitment of eIF4E and eIF4F to RNA.
Ifit1 and IFIT1 initially were suggested to associate directly with translation factors in an RNA-independent manner, effectively sequestering them from the active translation pool. However, this mechanism did not explain how IFIT1 preferentially could inhibit viral versus host mRNA translation. Recent studies show that Ifit1 and IFIT1 directly bind cap 0 RNA and prevent recruitment of translation factors (Habjan et al., 2013; Hyde et al., 2014; Kimura et al., 2013; Kumar et al., 2014). Because of its relatively greater affinity for RNA lacking 2′-O methylation, IFIT1 can out-compete eIF4E or eIF4F for binding, and thus remove cap 0 RNA from the actively translating pool (Kumar et al., 2014). Additionally, initiator and elongator tRNAs also compete with cap 0 RNA for IFIT1 and IFIT1B binding (Katibah et al., 2013; Katibah et al., 2014), suggesting that IFIT1 may inhibit protein synthesis of mRNA independent of cap methylation status, by sequestering tRNAs from the translating pool (Kumar et al., 2014). This study also demonstrated an association of IFIT1 with the 40S ribosomal subunit that was independent of RNA binding, suggesting yet another mechanism of general translation inhibition. The precise mechanism of translation inhibition may be influenced by the potential of IFIT1 to form homo- or heterodimers with other IFIT proteins (Habjan et al., 2013; Pichlmair et al., 2011). Although the functional relevance of IFIT oligomerization requires exploration, it is an attractive hypothesis by which the cell could use IFIT proteins to modulate translation control under different cellular conditions.
Mechanisms of viral evasion of Ifit1-mediated restriction
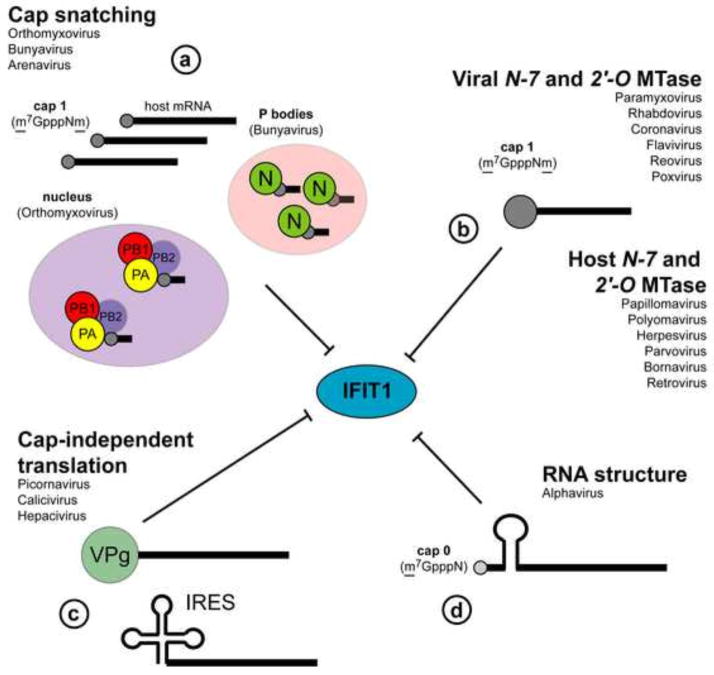
Viruses have evolved diverse mechanisms to circumvent IFIT1-mediated restriction including: (a) generating cap 1 structures on their RNA by cap-snatching, or using virally- or host-encoded 2′-O MTases; (b) using cap-independent means of translation; or (c) using RNA secondary structural motifs to antagonize Ifit1 binding (Fig 3).
Figure 3. Viral mechanisms of Ifit1 evasion and antagonism.
Viruses generate cap 1 structures through (a) cap-snatching (orthomyxoviurses, bunyaviruses, arenaviruses) whereby cap 1 is cleaved from host mRNA transcripts and used as a primer for viral RNA transcription. In contrast to orthomyxoviruses, which cap-snatch from mRNA in the nucleus, bunyaviruses associate with and sequester cap 1 containing nonsense-mRNA transcripts in cellular P bodies where N protein protects cap 1 from degradation. Some viruses mimic cap 1 structures via virally-encoded 2′-O MTases (flaviviruses, coronaviruses, rhabdoviruses, paramyxoviruses, reoviruses, and poxviruses). Other viruses (picornaviruses, caliciviruses, and hepaciviruses) circumvent Ifit1-mediated restriction by using non-canonical cap-like structures (VPg) at their 5′ end or IRES-mediated cap-independent translation (b). Alphaviruses antagonize Ifit1 function directly by inhibiting association with viral RNA through the generation of stable secondary structures in the 5′-UTR (c).
A. Viral 2′-O MTases
Many RNA viruses that replicate in the cytoplasm (including Flaviviruses, Coronaviruses, Poxviruses, Paramyxoviruses, Reoviruses, and Rhabdoviruses) encode their own viral 2′-O MTases, which catalyze the formation of cap structures on viral mRNA that mimic those present on host mRNAs. Mutant viruses encoding defective 2′-O MTases are sensitive to Ifit1-mediated restriction and attenuated in vivo (Daffis et al., 2010; Li et al., 2013; Menachery et al., 2014; Szretter et al., 2012; Zust et al., 2011). Although cap 1 structures are generated by virus-encoded MTases, the mechanism of cap methylation is distinct compared to eukaryotes (Fig 1C). Structural and biochemical analysis of the MTases from eukaryotic hosts and viruses have illustrated unique characteristics of viral 2′-O MTases, (Assenberg et al., 2007; Egloff et al., 2002; Egloff et al., 2007; Hodel et al., 1998), making them potential targets for drug development (reviewed in (Dong et al., 2008; Ferron et al., 2012)).
In contrast to cap formation in eukaryotic cells and by certain viruses (e.g., Poxviruses and Coronaviruses) where N-7 and 2′-O methylation is catalyzed by two enzymes, flaviviruses, reoviruses, and rhabdoviruses catalyze RNA methylation with a single viral enzyme (Assenberg et al., 2007; Bujnicki and Rychlewski, 2001, 2002; Ferron et al., 2002; Hercyk et al., 1988; Ray et al., 2006; Reinisch et al., 2000). The N-7 and 2′-O MTase activities of flaviviruses are encoded by the N-terminal domain of the NS5 gene (Ray et al., 2006). The NS5 MTase domain of flaviviruses possesses a Rossmann-like superfold and comprises a catalytic core of a seven-stranded β-sheet flanked by α-helices (reviewed in (Martin and McMillan, 2002; Schubert et al., 2003)). The catalytic tetrad (KDKE) of MTases is conserved in the flaviviruses, poxviruses, reoviruses, and coronaviruses (Assenberg et al., 2007; Decroly et al., 2011; Egloff et al., 2002; Egloff et al., 2007). Flavivirus MTase shows preferential binding for GTP, m7GTP, and m7GpppA within the cap-binding pocket. Substrates containing the first two native nucleotides (AG) of the flavivirus genome bind with higher affinity to the MTase than non-native nucleotide substrates, which may account for why these nucleotides are strictly conserved (Cleaves and Dubin, 1979; Egloff et al., 2007). m7GpppA and GpppA substrates also bind with different affinities, perhaps due to different modes of interaction of these substrates with the MTase (Egloff et al., 2002).
Reovirus capping and methylation is catalyzed by the λ2 protein, which forms pentamers that comprises part of the reovirus core (Koonin, 1993; Luongo et al., 1998; Reinisch et al., 2000). λ2 pentamers form a cylindrical structure through which viral mRNA passes and is capped and methylated at the N-7 and 2′-O position. The spatial arrangement of the catalytic sites within the λ2 pentamer indicates that capping, N-7- and 2′-O-methylation of viral RNA occurs sequentially as it shuttles from one end of the pentamer to the other. The presence of a flap at the terminus of the pentamer has been suggested to retain nascent viral RNA and ensure proper capping and methylation (Reinisch et al., 2000).
The N-7 and 2′-O MTase activity of coronaviruses are encoded by the viral NSP14 and NSP16 genes, respectively. While the coronavirus NSP14 N-7 MTase modifies nascent viral transcripts in a sequence-independent manner (Chen et al., 2013) the 2′-O MTase is sequence-specific, and requires a second viral protein (NSP10) as a cofactor (Chen et al., 2011; Decroly et al., 2008) for both RNA substrate and AdoMet binding.
The poxvirus 2′-O MTase VP39 catalyzes the formation of cap 1 structures. In contrast to reovirus λ2 and coronavirus NSP16, VP39 does not require oligmerization or cofactor binding for function (Hodel et al., 1998; Hodel et al., 1996; Shi et al., 1997). Viral RNA binding to VP39 is mediated by a hydrophobic pocket, which together with base stacking, is believed to accommodate substrates in a sequence-independent manner (Hodel et al., 1998; Hodel et al., 1996).
B. Cap-snatching by viruses
Orthomyxoviruses, bunyaviruses, and arenaviruses generate cap 1 structures by removing them from host mRNA transcripts via a mechanism called ‘cap-snatching’. During influenza virus infection, cap 1 structures are acquired from host nuclear mRNAs and used to prime viral RNA transcription. Both N-7 and 2′-O methylation of the cap are required for efficient transcriptional priming of influenza (Bouloy et al., 1980), which is mediated via PB2, the cap-binding protein of the influenza polymerase complex (PB1-PB2-PA) (Honda et al., 1999). Following cap 1 binding the viral polymerase complex cleaves the donor transcript 10 to 14 nucleotides downstream of the cap 1 structure (Dias et al., 2009; Li et al., 2001; Plotch et al., 1979; Plotch et al., 1981; Shi et al., 1995; Yuan et al., 2009). The polymerase complex preferentially cleaves at purine residues and requires additional sequence specificity, such as A/U at nucleotide positions 9–11, and yields a 3′-OH moiety (Kawakami et al., 1983; Plotch et al., 1981).
Bunyavirus cap-snatching exploits the host eukaryotic nonsense-mediate mRNA decay pathway to generate cap 1 primers for viral transcription (Cheng and Mir, 2012). Bunyavirus nucleocapsid (N) binds preferentially to host cap 1 mRNA with premature stop codons and then sequesters mRNA in processing (P) bodies within the cytoplasm where RNA degradation takes place (Mir et al., 2008). Association with N protects cap 1 from degradation by the host decapping machinery, and results in the generation of cap 1 RNA oligomers of ~180 nucleotides (Hopkins et al., 2013; Mir et al., 2008). The viral RNA-dependent RNA polymerase (L protein) then cleaves cap 1 oligomers at a G residue approximately 14 nucleotides downstream of cap 1 to yield ‘mature’ cap 1 oligomers that serve as primers for viral RNA transcription (Cheng and Mir, 2012; Jin and Elliott, 1993; Mir et al., 2008; Mir et al., 2010; Patterson et al., 1984).
Less is known about the cap-snatching mechanism used by arenaviruses (Lehmann et al., 2014; Morin et al., 2010; Raju et al., 1990; Reguera et al., 2010). Analogous to bunyaviruses, the arenavirus L protein has RNA-dependent RNA polymerase activity (Lukashevich et al., 1997; Vieth et al., 2004). L protein also encodes endonuclease and RNA-binding activity (Morin et al., 2010; Reguera et al., 2010) and is predicted to facilitate cap-binding and cleavage during cap-snatching through an undefined mechanism.
C. Cap-independent translation
Unlike eukaryotic mRNA and some viral RNA, which have cap 1 structures and use cap-dependent translation mechanisms, other viruses have a 5′ covalently-linked viral protein, VPg (picornaviruses and caliciviruses) and/or an internal ribosomal entry site (IRES; picornaviruses and hepaciviruses) within the 5′-untranslated region (UTR) to facilitate cap-independent translation. In contrast to canonical cap-mediated translation where 43S pre-initiation complex association with the mRNA cap structure is followed by ribosomal scanning and 48S and 60S association, in IRES-mediated translation, the 43S complex binds directly upstream of the IRES and does not require ribosomal scanning or certain translation initiation factors (eIF1, eIF1A, and eIF4E) (reviewed in (Jackson et al., 2010; Pestova et al., 2001)). Viruses lacking 2′-O methylation on their viral mRNA (e.g., picornaviruses, caliciviruses, and hepaciviruses) may use cap-independent translation mechanisms to overcome Ifit1 restriction. In theory, this might occur in one of two ways: (a) the VPg and/or IRES structures sterically hinder Ifit1 binding or (b) the IRES bypasses the step at which IFIT1 inhibits eIF3-dependent translation.
D. RNA structure-mediated antagonism
Alphaviruses are RNA viruses that replicate in the cytoplasm, translate via a cap-dependent mechanism, and yet lack a virally encoded 2′-O-methyltransferase or cap-snatching mechanism, and thus should be restricted by IFIT1. Alphaviruses have a defined cap 0 structure on the 5′ end of their viral genomic and subgenomic RNA (Hefti et al., 1975; Pettersson et al., 1980). Although previous studies had shown that mutations in the Venezuelan equine encephalitis virus (VEEV) 5′-UTR (G→A at position 3) were attenuating (Kinney et al., 1993; Kinney et al., 1989) and resulted in enhanced sensitivity to type I IFN treatment (White et al., 2001), the basis for this was unknown. Analogously, mutations that altered the pathogenicity and IFN-sensitivity of other alphaviruses (e.g., Sindbis and Semliki Forest viruses) also mapped to the 5′-UTR (Klimstra et al., 1999; Kobiler et al., 1999; Kuhn et al., 1992; Logue et al., 2008). A recent study explains these earlier findings as it showed that RNA secondary structure in alphavirus 5′-UTR antagonizes Ifit1 binding and antiviral activity (Hyde et al., 2014). Mutations within the 5′-UTR that affect stable RNA structural elements enabled restriction by or antagonism of Ifit1 in vitro and in vivo by altering binding of Ifit1 to viral RNA. Although this mechanism has not yet been described for other viruses, stable RNA structures are commonly found within the 5′-UTR of many viruses, which might impact Ifit1 recognition in other viral systems.
Conclusions
In higher eukaryotes, detection of viral RNA is critical for initiating and establishing an antiviral state in cells. Cap 0 structures on viral RNA can act as PAMPs that are sensed and restricted by the IFN-induced gene, IFIT1. In contrast to other RNA sensors (TLRs and RLRs) that induce signaling cascades that lead to the expression of type I IFN and ISGs, Ifit1 appears to function predominantly as an antiviral effector molecule. The evolution of multiple mechanisms of antagonism by different families of RNA and DNA viruses indicates the importance of Ifit1 as a key RNA sensor and antiviral molecule. Pathogenic viruses have evolved ways to produce mRNA with 5′-ends that mimic host cellular mRNAs, including viral RNA with N-7 and 2′-O methylation through several independent strategies (encoding capping machinery, ‘cap-snatching’, or using host capping machinery) or bypassing cap-dependent translation to use other modalities (IRES-dependent) for protein synthesis. The finding that different IFIT proteins exhibit varying affinities for distinct RNA ligands suggests that IFIT genes may recognize additional viral PAMPs, which have yet to be identified. The identification of Ifit1 as a key RNA sensor and antiviral effector and the importance of cap 1 structures in abrogating Ifit1-mediated restriction has provided a rationale for the development of novel antiviral interventions, including live attenuated vaccines (with 2′-O-methylation or RNA structure mutants) and small molecule inhibitors of viral 2′-O MTases.
Acknowledgments
NIH grants U19 AI083019, U19 AI106772, R01 AI104972, and R01 AI104002 supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas YM, Pichlmair A, Gorna MW, Superti-Furga G, Nagar B. Structural basis for viral 5′-PPP-RNA recognition by human IFIT proteins. Nature. 2013;494:60–64. doi: 10.1038/nature11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejeva J, Norsted H, Habjan M, Thiel V, Goodbourn S, Randall RE. ISG56/IFIT1 is primarily responsible for interferon-induced changes to patterns of parainfluenza virus type 5 transcription and protein synthesis. The Journal of general virology. 2013;94:59–68. doi: 10.1099/vir.0.046797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenberg R, Ren J, Verma A, Walter TS, Alderton D, Hurrelbrink RJ, Fuller SD, Bressanelli S, Owens RJ, Stuart DI, Grimes JM. Crystal structure of the Murray Valley encephalitis virus NS5 methyltransferase domain in complex with cap analogues. The Journal of general virology. 2007;88:2228–2236. doi: 10.1099/vir.0.82757-0. [DOI] [PubMed] [Google Scholar]
- Belanger F, Stepinski J, Darzynkiewicz E, Pelletier J. Characterization of hMTr1, a human Cap1 2′-O-ribose methyltransferase. The Journal of biological chemistry. 2010;285:33037–33044. doi: 10.1074/jbc.M110.155283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Current opinion in cell biology. 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Both GW, Furuichi Y, Muthukrishnan S, Shatkin AJ. Ribosome binding to reovirus mRNA in protein synthesis requires 5′ terminal 7-methylguanosine. Cell. 1975;6:185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Bouloy M, Plotch SJ, Krug RM. Both the 7-methyl and the 2′-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nature reviews Immunology. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnicki JM, Rychlewski L. Reassignment of specificities of two cap methyltransferase domains in the reovirus lambda 2 protein. Genome biology. 2001;2:RESEARCH0038. doi: 10.1186/gb-2001-2-9-research0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnicki JM, Rychlewski L. In silico identification, structure prediction and phylogenetic analysis of the 2′-O-ribose (cap 1) methyltransferase domain in the large structural protein of ssRNA negative-strand viruses. Protein engineering. 2002;15:101–108. doi: 10.1093/protein/15.2.101. [DOI] [PubMed] [Google Scholar]
- Chen Y, Su C, Ke M, Jin X, Xu L, Zhang Z, Wu A, Sun Y, Yang Z, Tien P, Ahola T, Liang Y, Liu X, Guo D. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2′-O-methylation by nsp16/nsp10 protein complex. PLoS pathogens. 2011;7:e1002294. doi: 10.1371/journal.ppat.1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tao J, Sun Y, Wu A, Su C, Gao G, Cai H, Qiu S, Wu Y, Ahola T, Guo D. Structure-function analysis of severe acute respiratory syndrome coronavirus RNA cap guanine-N7-methyltransferase. Journal of virology. 2013;87:6296–6305. doi: 10.1128/JVI.00061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng E, Mir MA. Signatures of host mRNA 5′ terminus for efficient hantavirus cap snatching. Journal of virology. 2012;86:10173–10185. doi: 10.1128/JVI.05560-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TD, Cianci C, Hagen M, Terry B, Matthews JT, Krystal M, Colonno RJ. Biochemical studies on capped RNA primers identify a class of oligonucleotide inhibitors of the influenza virus RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2372–2376. doi: 10.1073/pnas.91.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaves GR, Dubin DT. Methylation status of intracellular dengue type 2 40 S RNA. Virology. 1979;96:159–165. doi: 10.1016/0042-6822(79)90181-8. [DOI] [PubMed] [Google Scholar]
- D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends in biochemical sciences. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H, Thiel V, Sen GC, Fensterl V, Klimstra WB, Pierson TC, Buller RM, Gale M, Jr, Shi PY, Diamond MS. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E, Debarnot C, Ferron F, Bouvet M, Coutard B, Imbert I, Gluais L, Papageorgiou N, Sharff A, Bricogne G, Ortiz-Lombardia M, Lescar J, Canard B. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2′-Omethyltransferase nsp10/nsp16 complex. PLoS pathogens. 2011;7:e1002059. doi: 10.1371/journal.ppat.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E, Imbert I, Coutard B, Bouvet M, Selisko B, Alvarez K, Gorbalenya AE, Snijder EJ, Canard B. Coronavirus nonstructural protein 16 is a cap-0 binding enzyme possessing (nucleoside-2′O)-methyltransferase activity. Journal of virology. 2008;82:8071–8084. doi: 10.1128/JVI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A, Bouvier D, Crepin T, McCarthy AA, Hart DJ, Baudin F, Cusack S, Ruigrok RW. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–918. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang B, Shi PY. Flavivirus methyltransferase: a novel antiviral target. Antiviral research. 2008;80:1–10. doi: 10.1016/j.antiviral.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Hartmuth K, Luhrmann R. Modified nucleotides at the 5′ end of human U2 snRNA are required for spliceosomal E-complex formation. RNA. 2004;10:1925–1933. doi: 10.1261/rna.7186504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. The EMBO journal. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Decroly E, Malet H, Selisko B, Benarroch D, Ferron F, Canard B. Structural and functional analysis of methylation and 5′-RNA sequence requirements of short capped RNAs by the methyltransferase domain of dengue virus NS5. Journal of molecular biology. 2007;372:723–736. doi: 10.1016/j.jmb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ensinger MJ, Moss B. Modification of the 5′ terminus of mRNA by an RNA (guanine-7-)-methyltransferase from HeLa cells. The Journal of biological chemistry. 1976;251:5283–5291. [PubMed] [Google Scholar]
- Feng F, Yuan L, Wang YE, Crowley C, Lv Z, Li J, Liu Y, Cheng G, Zeng S, Liang H. Crystal structure and nucleotide selectivity of human IFIT5/ISG58. Cell research. 2013;23:1055–1058. doi: 10.1038/cr.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, Sen GC. The ISG56/IFIT1 gene family. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2011;31:71–78. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron F, Decroly E, Selisko B, Canard B. The viral RNA capping machinery as a target for antiviral drugs. Antiviral research. 2012;96:21–31. doi: 10.1016/j.antiviral.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron F, Longhi S, Henrissat B, Canard B. Viral RNA-polymerases -- a predicted 2′-O-ribose methyltransferase domain shared by all Mononegavirales. Trends in biochemical sciences. 2002;27:222–224. doi: 10.1016/s0968-0004(02)02091-1. [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nature reviews Molecular cell biology. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nature reviews Molecular cell biology. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends in biochemical sciences. 1991;16:173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Guo J, Hui DJ, Merrick WC, Sen GC. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. The EMBO journal. 2000a;19:6891–6899. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Peters KL, Sen GC. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology. 2000b;267:209–219. doi: 10.1006/viro.1999.0135. [DOI] [PubMed] [Google Scholar]
- Habjan M, Hubel P, Lacerda L, Benda C, Holze C, Eberl CH, Mann A, Kindler E, Gil-Cruz C, Ziebuhr J, Thiel V, Pichlmair A. Sequestration by IFIT1 Impairs Translation of 2′O-unmethylated Capped RNA. PLoS pathogens. 2013;9:e1003663. doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haline-Vaz T, Silva TC, Zanchin NI. The human interferon-regulated ISG95 protein interacts with RNA polymerase II and shows methyltransferase activity. Biochemical and biophysical research communications. 2008;372:719–724. doi: 10.1016/j.bbrc.2008.05.137. [DOI] [PubMed] [Google Scholar]
- Hefti E, Bishop DH, Dubin DT, Stollar V. 5′ nucleotide sequence of sindbis viral RNA. Journal of virology. 1975;17:149–159. doi: 10.1128/jvi.17.1.149-159.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercyk N, Horikami SM, Moyer SA. The vesicular stomatitis virus L protein possesses the mRNA methyltransferase activities. Virology. 1988;163:222–225. doi: 10.1016/0042-6822(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Hodel AE, Gershon PD, Quiocho FA. Structural basis for sequence-nonspecific recognition of 5′-capped mRNA by a cap-modifying enzyme. Molecular cell. 1998;1:443–447. doi: 10.1016/s1097-2765(00)80044-1. [DOI] [PubMed] [Google Scholar]
- Hodel AE, Gershon PD, Shi X, Quiocho FA. The 1.85 A structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends. Cell. 1996;85:247–256. doi: 10.1016/s0092-8674(00)81101-0. [DOI] [PubMed] [Google Scholar]
- Honda A, Mizumoto K, Ishihama A. Two separate sequences of PB2 subunit constitute the RNA cap-binding site of influenza virus RNA polymerase. Genes to cells : devoted to molecular & cellular mechanisms. 1999;4:475–485. doi: 10.1046/j.1365-2443.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- Hopkins KC, McLane LM, Maqbool T, Panda D, Gordesky-Gold B, Cherry S. A genome-wide RNAi screen reveals that mRNA decapping restricts bunyaviral replication by limiting the pools of Dcp2-accessible targets for cap-snatching. Genes & development. 2013;27:1511–1525. doi: 10.1101/gad.215384.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DJ, Bhasker CR, Merrick WC, Sen GC. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. The Journal of biological chemistry. 2003;278:39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- Hui DJ, Terenzi F, Merrick WC, Sen GC. Mouse p56 blocks a distinct function of eukaryotic initiation factor 3 in translation initiation. The Journal of biological chemistry. 2005;280:3433–3440. doi: 10.1074/jbc.M406700200. [DOI] [PubMed] [Google Scholar]
- Hyde JL, Gardner CL, Kimura T, White JP, Liu G, Trobaugh DW, Huang C, Tonelli M, Paessler S, Takeda K, Klimstra WB, Amarasinghe GK, Diamond MS. A viral RNA structural element alters host recognition of nonself RNA. Science. 2014;343:783–787. doi: 10.1126/science.1248465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature reviews Molecular cell biology. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Elliott RM. Characterization of Bunyamwera virus S RNA that is transcribed and replicated by the L protein expressed from recombinant vaccinia virus. Journal of virology. 1993;67:1396–1404. doi: 10.1128/jvi.67.3.1396-1404.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katibah GE, Lee HJ, Huizar JP, Vogan JM, Alber T, Collins K. tRNA Binding, Structure, and Localization of the Human Interferon-Induced Protein IFIT5. Molecular cell. 2013 doi: 10.1016/j.molcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katibah GE, Qin Y, Sidote DJ, Yao J, Lambowitz AM, Collins K. Broad and adaptable RNA structure recognition by the human interferon-induced tetratricopeptide repeat protein IFIT5. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12025–12030. doi: 10.1073/pnas.1412842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Mizumoto K, Ishihama A. RNA polymerase of influenza virus. IV. Catalytic properties of the capped RNA endonuclease associated with the RNA polymerase. Nucleic acids research. 1983;11:3637–3649. doi: 10.1093/nar/11.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Katoh H, Kayama H, Saiga H, Okuyama M, Okamoto T, Umemoto E, Matsuura Y, Yamamoto M, Takeda K. Ifit1 Inhibits Japanese Encephalitis Virus Replication through Binding to 5′ Capped 2′-O Unmethylated RNA. Journal of virology. 2013;87:9997–10003. doi: 10.1128/JVI.00883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Kinney RM, Chang GJ, Tsuchiya KR, Sneider JM, Roehrig JT, Woodward TM, Trent DW. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. Journal of virology. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney RM, Johnson BJ, Welch JB, Tsuchiya KR, Trent DW. The full-length nucleotide sequences of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and its attenuated vaccine derivative, strain TC-83. Virology. 1989;170:19–30. doi: 10.1016/0042-6822(89)90347-4. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Spleiss O, Li H, Taniguchi T, Kimura H, Nomura Y, Gebicke-Haerter PJ. Lipopolysaccharide-induced switch between retinoid receptor (RXR) alpha and glucocorticoid attenuated response gene (GARG)-16 messenger RNAs in cultured rat microglia. Journal of neuroscience research. 2001;64:553–563. doi: 10.1002/jnr.1107. [DOI] [PubMed] [Google Scholar]
- Klimstra WB, Ryman KD, Bernard KA, Nguyen KB, Biron CA, Johnston RE. Infection of neonatal mice with sindbis virus results in a systemic inflammatory response syndrome. Journal of virology. 1999;73:10387–10398. doi: 10.1128/jvi.73.12.10387-10398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiler D, Rice CM, Brodie C, Shahar A, Dubuisson J, Halevy M, Lustig S. A single nucleotide change in the 5′ noncoding region of Sindbis virus confers neurovirulence in rats. Journal of virology. 1999;73:10440–10446. doi: 10.1128/jvi.73.12.10440-10446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. The Journal of general virology. 1993;74 (Pt 4):733–740. doi: 10.1099/0022-1317-74-4-733. [DOI] [PubMed] [Google Scholar]
- Kuge H, Brownlee GG, Gershon PD, Richter JD. Cap ribose methylation of c-mos mRNA stimulates translation and oocyte maturation in Xenopus laevis. Nucleic acids research. 1998;26:3208–3214. doi: 10.1093/nar/26.13.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge H, Richter JD. Cytoplasmic 3′ poly(A) addition induces 5′ cap ribose methylation: implications for translational control of maternal mRNA. The EMBO journal. 1995;14:6301–6310. doi: 10.1002/j.1460-2075.1995.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn RJ, Griffin DE, Zhang H, Niesters HG, Strauss JH. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. Journal of virology. 1992;66:7121–7127. doi: 10.1128/jvi.66.12.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sweeney TR, Skabkin MA, Skabkina OV, Hellen CU, Pestova TV. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1- and 5′ppp- mRNAs. Nucleic acids research. 2014;42:3228–3245. doi: 10.1093/nar/gkt1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends in biochemical sciences. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Langberg SR, Moss B. Post-transcriptional modifications of mRNA. Purification and characterization of cap I and cap II RNA (nucleoside-2′-)-methyltransferases from HeLa cells. The Journal of biological chemistry. 1981;256:10054–10060. [PubMed] [Google Scholar]
- Lehmann M, Pahlmann M, Jerome H, Busch C, Lelke M, Gunther S. Role of the C terminus of Lassa virus L protein in viral mRNA synthesis. Journal of virology. 2014;88:8713–8717. doi: 10.1128/JVI.00652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Rao P, Krug RM. The active sites of the influenza cap-dependent endonuclease are on different polymerase subunits. The EMBO journal. 2001;20:2078–2086. doi: 10.1093/emboj/20.8.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Dong H, Li XF, Xie X, Zhao H, Deng YQ, Wang XY, Ye Q, Zhu SY, Wang HJ, Zhang B, Leng QB, Zuest R, Qin ED, Qin CF, Shi PY. Rational design of a flavivirus vaccine by abolishing viral RNA 2′-O methylation. Journal of virology. 2013;87:5812–5819. doi: 10.1128/JVI.02806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang YB, Liu TK, Gui JF. Lineage-specific expansion of IFIT gene family: an insight into coevolution with IFN gene family. PloS one. 2013;8:e66859. doi: 10.1371/journal.pone.0066859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue CH, Sheahan BJ, Atkins GJ. The 5′ untranslated region as a pathogenicity determinant of Semliki Forest virus in mice. Virus genes. 2008;36:313–321. doi: 10.1007/s11262-008-0209-1. [DOI] [PubMed] [Google Scholar]
- Lukashevich IS, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol ST, Salvato MS. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. The Journal of general virology. 1997;78 (Pt 3):547–551. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo CL, Contreras CM, Farsetta DL, Nibert ML. Binding site for S-adenosyl-L-methionine in a central region of mammalian reovirus lambda2 protein. Evidence for activities in mRNA cap methylation. The Journal of biological chemistry. 1998;273:23773–23780. doi: 10.1074/jbc.273.37.23773. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wei Y, Zhang X, Zhang Y, Cai H, Zhu Y, Shilo K, Oglesbee M, Krakowka S, Whelan SP, Li J. mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo. Journal of virology. 2014;88:2913–2926. doi: 10.1128/JVI.03420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Current opinion in structural biology. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- Menachery VD, Yount BL, Jr, Josset L, Gralinski LE, Scobey T, Agnihothram S, Katze MG, Baric RS. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2′-o-methyltransferase activity. Journal of virology. 2014;88:4251–4264. doi: 10.1128/JVI.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir MA, Duran WA, Hjelle BL, Ye C, Panganiban AT. Storage of cellular 5′ mRNA caps in P bodies for viral cap-snatching. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19294–19299. doi: 10.1073/pnas.0807211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir MA, Sheema S, Haseeb A, Haque A. Hantavirus nucleocapsid protein has distinct m7G cap- and RNA-binding sites. The Journal of biological chemistry. 2010;285:11357–11368. doi: 10.1074/jbc.M110.102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin B, Coutard B, Lelke M, Ferron F, Kerber R, Jamal S, Frangeul A, Baronti C, Charrel R, de Lamballerie X, Vonrhein C, Lescar J, Bricogne G, Gunther S, Canard B. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS pathogens. 2010;6:e1001038. doi: 10.1371/journal.ppat.1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JL, Holloway B, Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. Journal of virology. 1984;52:215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Schwer B, Shuman S. Interactions between fission yeast Cdk9, its cyclin partner Pch1, and mRNA capping enzyme Pct1 suggest an elongation checkpoint for mRNA quality control. The Journal of biological chemistry. 2003;278:7180–7188. doi: 10.1074/jbc.M211713200. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson RF, Soderlund H, Kaariainen L. The nucleotide sequences of the 5′-terminal T1 oligonucleotides of Semliki-Forest-virus 42-S and 26-S RNAs are different. European journal of biochemistry / FEBS. 1980;105:435–443. doi: 10.1111/j.1432-1033.1980.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Lassnig C, Eberle CA, Gorna MW, Baumann CL, Burkard TR, Burckstummer T, Stefanovic A, Krieger S, Bennett KL, Rulicke T, Weber F, Colinge J, Muller M, Superti-Furga G. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nature immunology. 2011;12:624–630. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- Plotch SJ, Bouloy M, Krug RM. Transfer of 5′-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch SJ, Bouloy M, Ulmanen I, Krug RM. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Raju R, Raju L, Hacker D, Garcin D, Compans R, Kolakofsky D. Nontemplated bases at the 5′ ends of Tacaribe virus mRNAs. Virology. 1990;174:53–59. doi: 10.1016/0042-6822(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, Shi PY. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. Journal of virology. 2006;80:8362–8370. doi: 10.1128/JVI.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera J, Weber F, Cusack S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS pathogens. 2010;6:e1001101. doi: 10.1371/journal.ppat.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch KM, Nibert ML, Harrison SC. Structure of the reovirus core at 3.6 A resolution. Nature. 2000;404:960–967. doi: 10.1038/35010041. [DOI] [PubMed] [Google Scholar]
- Schroeder SC, Schwer B, Shuman S, Bentley D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes & development. 2000;14:2435–2440. doi: 10.1101/gad.836300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends in biochemical sciences. 2003;28:329–335. doi: 10.1016/S0968-0004(03)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shatkin AJ. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985;40:223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Shi L, Summers DF, Peng Q, Galarz JM. Influenza A virus RNA polymerase subunit PB2 is the endonuclease which cleaves host cell mRNA and functions only as the trimeric enzyme. Virology. 1995;208:38–47. doi: 10.1006/viro.1995.1127. [DOI] [PubMed] [Google Scholar]
- Shi X, Bernhardt TG, Wang SM, Gershon PD. The surface region of the bifunctional vaccinia RNA modifying protein VP39 that interfaces with Poly(A) polymerase is remote from the RNA binding cleft used for its mRNA 5′ cap methylation function. The Journal of biological chemistry. 1997;272:23292–23302. doi: 10.1074/jbc.272.37.23292. [DOI] [PubMed] [Google Scholar]
- Smietanski M, Werner M, Purta E, Kaminska KH, Stepinski J, Darzynkiewicz E, Nowotny M, Bujnicki JM. Structural analysis of human 2′-O-ribose methyltransferases involved in mRNA cap structure formation. Nature communications. 2014:5. doi: 10.1038/ncomms4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Herschman HR. The glucocorticoid attenuated response genes GARG-16, GARG-39, and GARG-49/IRG2 encode inducible proteins containing multiple tetratricopeptide repeat domains. Archives of biochemistry and biophysics. 1996;330:290–300. doi: 10.1006/abbi.1996.0256. [DOI] [PubMed] [Google Scholar]
- Szretter KJ, Daniels BP, Cho H, Gainey MD, Yokoyama WM, Gale M, Jr, Virgin HW, Klein RS, Sen GC, Diamond MS. 2′-O methylation of the viral mRNA cap by West Nile virus evades ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS pathogens. 2012;8:e1002698. doi: 10.1371/journal.ppat.1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi F, Pal S, Sen GC. Induction and mode of action of the viral stress-inducible murine proteins, P56 and P54. Virology. 2005;340:116–124. doi: 10.1016/j.virol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Terlecky SR, Nuttley WM, McCollum D, Sock E, Subramani S. The Pichia pastoris peroxisomal protein PAS8p is the receptor for the C-terminal tripeptide peroxisomal targeting signal. The EMBO journal. 1995;14:3627–3634. doi: 10.1002/j.1460-2075.1995.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D, Struhl K. Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8- Tup1 corepressor complex to differentially regulated promoters. Genes & development. 1995;9:821–831. doi: 10.1101/gad.9.7.821. [DOI] [PubMed] [Google Scholar]
- Varela M, Diaz-Rosales P, Pereiro P, Forn-Cuni G, Costa MM, Dios S, Romero A, Figueras A, Novoa B. Interferon-induced genes of the expanded IFIT family show conserved antiviral activities in non-mammalian species. PloS one. 2014;9:e100015. doi: 10.1371/journal.pone.0100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S, Gershowitz A, Moss B. Purification and characterization of mRNA guanylyltransferase from HeLa cell nuclei. The Journal of biological chemistry. 1980;255:2829–2834. [PubMed] [Google Scholar]
- Venkatesan S, Moss B. Donor and acceptor specificities of HeLa cell mRNA guanylyltransferase. The Journal of biological chemistry. 1980;255:2835–2842. [PubMed] [Google Scholar]
- Vieth S, Torda AE, Asper M, Schmitz H, Gunther S. Sequence analysis of L RNA of Lassa virus. Virology. 2004;318:153–168. doi: 10.1016/j.virol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Wang C, Pflugheber J, Sumpter R, Jr, Sodora DL, Hui D, Sen GC, Gale M., Jr Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. Journal of virology. 2003;77:3898–3912. doi: 10.1128/JVI.77.7.3898-3912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LJ, Wang JG, Davis NL, Johnston RE. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. Journal of virology. 2001;75:3706–3718. doi: 10.1128/JVI.75.8.3706-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Mizumoto K, Kaziro Y. Association of an RNA 5′-triphosphatase activity with RNA guanylyltransferase partially purified from rat liver nuclei. The EMBO journal. 1983;2:611–615. doi: 10.1002/j.1460-2075.1983.tb01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Okabe T, Doi R, Shimmi O, Arisawa M, Yamada-Okabe H. Isolation and characterization of a human cDNA for mRNA 5′-capping enzyme. Nucleic acids research. 1998;26:1700–1706. doi: 10.1093/nar/26.7.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liang H, Zhou Q, Li Y, Chen H, Ye W, Chen D, Fleming J, Shu H, Liu Y. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell research. 2012 doi: 10.1038/cr.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, Lv Z, Ge R, Li X, Deng T, Fodor E, Rao Z, Liu Y. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–913. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wei Y, Zhang X, Cai H, Niewiesk S, Li J. Rational design of human metapneumovirus live attenuated vaccine candidates by inhibiting viral mRNA cap methyltransferase. Journal of virology. 2014;88:11411–11429. doi: 10.1128/JVI.00876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS, Siddell SG, Ludewig B, Thiel V. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nature immunology. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R, Dong H, Li XF, Chang DC, Zhang B, Balakrishnan T, Toh YX, Jiang T, Li SH, Deng YQ, Ellis BR, Ellis EM, Poidinger M, Zolezzi F, Qin CF, Shi PY, Fink K. Rational design of a live attenuated dengue vaccine: 2′-o-methyltransferase mutants are highly attenuated and immunogenic in mice and macaques. PLoS pathogens. 2013;9:e1003521. doi: 10.1371/journal.ppat.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]