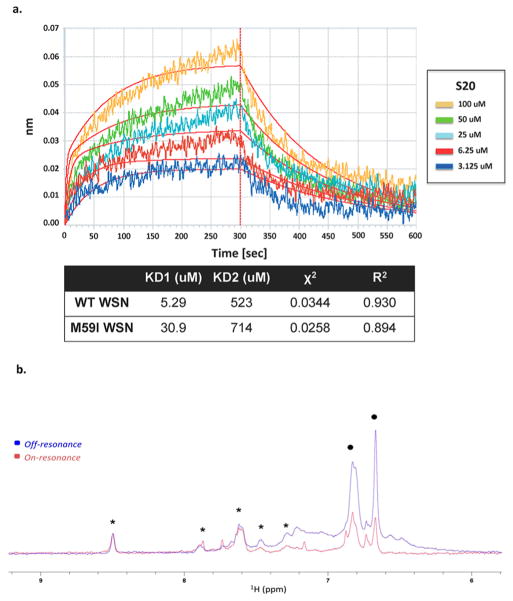
Figure 6.
S20 directly binds to the influenza virus hemagglutinin protein. (a) Biolayer interferometry was used to assay the binding of the small molecule S20 to purified WSN HA protein. The association and dissociation curves of increasing concentrations of S20 binding to WT WSN HA are shown. A negative control of buffer (PBS, 0.1% Tween-20, 10% DMSO) containing no HA was run for each experiment. The affinity of S20 for HA–WT and HA–M59I was calculated and represented as two separate dissociation constants for the high-affinity specific interaction (KD1) and the low-affinity nonspecific binding (KD2). A χ2 of 0.0344 and an R2 of 0.93 indicate that the binding data fit to this 2:1 binding model (i.e., two binding events on HA). (b) NMR binding of S20 to WT WSN HA via saturation transfer difference (STD). Overlay of the aromatic region of on-resonance (0.5 ppm, red) and off-resonance (20 ppm, blue) 1H NMR spectra of 75 μM compound S20 (resonances between 6.6 and 7.0 ppm, black dots) and 75 μM of a unrelated small molecule used as negative control (resonances between 8.5 and 7.25 ppm, asterisks) in PBS, pH 7.4 (+10% D2O) in the presence of 7.5 μM WT WSN HA. Selective attenuation (saturation) of S20 protons (indicated with dots) is evident, whereas the resonances of the negative control compound (indicated with asterisks) appear unperturbed.