Abstract
Animal models are commonly used throughout research laboratories as a tool to accomplish what would normally be considered impractical in a pathogen's native host. Milk collection from animals allows scientists the opportunity to study many aspects of reproduction including vertical transmission, passive immunity, mammary gland biology, and lactation. Obtaining adequate volumes of milk for these studies is a challenging task, especially from small animal models. Here we illustrate an inexpensive and facile method for milk collection in mice and Reeves’ muntjac deer that does not require specialized equipment or extensive training.
Keywords: mouse, milk, murine, muntjac, doe
Introduction
Animal models provide insight into disease pathology that cannot be gained by in vitro analysis. To provide the most efficacious results, it is important to use an animal model that is closely related to the disease and species of interest. For example, the Reeves’ muntjac (Muntiacus reevesi), a small Asian deer (1, 2), and transgenic mice expressing the cervid prion protein (CerTgPrP) (3) are useful animal models for cervid species. Both species are polyestrous, allowing year-round breeding, and therefore a consistent source for pregnancy-related tissues and fluids to study specific mechanisms in cervid biology. Studies of milk have a vast array of applications that are more simply (and inexpensively) accomplished in animal models than in humans. Researchers could investigate milk and colostrum as a potential source of 1) infectious disease transmission, 2) immunoglobulins transferred from mother to offspring in the development of passive immunity (4) and 3) lactoferrin, a protein found in human breast milk involved in passive immunity that researchers are currently attempting to commercially produce (5).
Collecting a substantial amount of milk from small animals can prove to be a difficult task. Rogers (6) proposed an approach to collect milk from rats, which was subsequently used in mice. DePeters and Hovey proposed two methods for milk collection, one using a manually-generated vacuum produced by a rubber pipette bulb attached to a Pasteur pipette, and a second requiring the construction of a milking unit, which is then attached to a vacuum source (such as a faucet) to harvest mouse milk (7). Here we propose a simple, low cost method for collecting milk from both mice and Reeves’ muntjac deer, which requires only readily available laboratory equipment and basic technical skills. Our method yields sufficient volumes of milk for various applications.
Mouse Milking Protocol
1. Separation of the dam from offspring
1.1 Select the dam to be milked. Choosing a dam with a litter of 4 or more pups that is 8-12 days post parturition provides maximal milk collection— although collection is possible at any time point post parturition up to 21 days. (Table 1)
Table 1.
Approximate Milk Yield Based on Litter Size, Time Post Parturition, and Oxytocin Dosage
| Days post parturition | Litter size | Approx. milk yield (μL) | Oxytocin dosage (IU/kg) |
|---|---|---|---|
| 1 | 4 | >1 | 0.1 |
| 3 | 7 | 180 | 2 |
| 4 | 8 | 100 | 2 |
| 7 | 6 | 430 | 2 |
| 8 | 4 | 120 | 2 |
| 8 | 8 | 200 | 2 |
| 9 | 3 | 300 | 2 |
| 9 | 6 | 300 | 2 |
| 9 | 8 | 200 | 2 |
| 9 | 9 | 420 | 2 |
| 10 | 2 | 100 | 2 |
| 12 | 4 | 400 | 2 |
| 12 | 7 | 200 | 2 |
| 13 | 2 | 280 | 2 |
| 13 | 7 | 190 | 2 |
| 14 | 4 | 100 | 2 |
| 16 | 4 | 200 | 2 |
1.2 Separate the dam from her litter at least 2 hours before milking.
1.2.1 Should one desire to milk more than one dam in a given time period, it is acceptable to house the separated dams in the same cage with adequate food and water supply.
2. Administration of oxytocin, anesthesia and eye lubricant
2.1 Administer 0.1 ml of oxytocin (2IU/kg) intraperitoneally (IP). Oxytocin is a hormone that acts on the mammary glands of lactating females to stimulate the release of milk. Oxytocin can be acquired from a veterinarian.
2.2 Administer the anesthetic IP. Our laboratory uses an IACUC approved ketamine/xylazine mixture of the following dosage:
■ ketamine 80-100 mg/kg
■ xylazine 5-10 mg/kg
Ex: The anesthetic used for our dams contains 0.1 ml xylazine, 8.9 ml H2O, and 1.0 ml ketamine
2.2.1. If milking more than one dam, it is necessary to stagger oxytocin and anesthetic injections due to the short half life of oxytocin and sedation times for ketamine and xylazine. 0.25 ml total anesthetic will sedate a mouse for approximately 20-30 minutes, which should provide adequate time for milk collection.
2.3 Apply a small amount of eye lubricant to the corner of each eye and spread to prevent the mouse's eyes from drying out while under anesthesia.
3. Milk collection
Note: The milk collection is most easily performed with two researchers: one researcher to hold the anesthetized mouse while manually expressing the milk (referred to as R1,) and one researcher to collect the milk (R2.) This method can also be performed with one person, if the mouse is secured on a flat surface to avoid harm.
3.1 R2 should be equipped with a P-200 pipetman, a clean pipette tip for each mouse, and a container to hold the milk, such as a 1.5 ml eppendorf tube or a 1.2 ml cryovial. (Table of Materials.)
3.2 As the oxytocin begins to take effect, milk letdown will become visible in the mouse's mammary area. R1 expresses milk from the teat by using the thumb and forefinger to gently massage and squeeze the mammary tissue in an upward motion until a visible bead of milk begins to form at the base of the teat.
3.3 R2 will press the P-200 pipetman plunger to its first stop to release air out of the pipette tip and prepare for milk collection.
3.4 Position the pipette tip at the top of the drop of milk, and gently pull the milk into the pipette tip by slowly releasing pressure on the plunger. Take care not to put the tip too close to the teat or the skin. It is not necessary to completely release the plunger as this will pull the milk all the way to the top of the pipette tip and will make it difficult to expel the often viscous milk into the holding container.
3.5 Expel the milk into the container by using the thumb to press the plunger past the first stop. To facilitate expulsion of small volumes, press the pipette tip against the side of the tube so that no liquid is lost.
3.6 R1 will continue to express milk from each teat, moving in either a clockwise or counterclockwise direction so as not to skip any teats. It is both acceptable and useful to come back to and continually express milk from the same teat should it produce more milk than another.
4. Anesthetic Reversal in the Dam
4.1 In most cases, it is common for the dam to begin to wake up during the milking process. If this is not the case, we suggest placing the dam in a cage with a heat source, such as a heating lamp, until she begins to wake up.
4.1.1 Once the dam has begun to wake up, it will be necessary to keep close observation of her activity until she is able to walk about her cage on her own. At this point, it is acceptable to put her back into her original cage with her litter. No ill effects have been noted with respect to the pups’ ability to feed after milk collection.
Muntjac Milking Protocol
1. Separation of the doe from offspring
1.1 Select the doe to be milked. Reeves’ muntjac deer generally give birth to one fawn (2). The doe can be milked the day after birthing to allow the fawn an opportunity to gain access to essential nutrients in colostrum (8).
1.2 The fawn usually hides and does not reappear during the procedure, therefore separating the mother-offspring pair is not necessary.
2. Administration of oxytocin, anesthesia and eye lubricant
2.1 Administer 0.5 ml of oxytocin (10 IU/kg) intramuscularly (IM). Oxytocin is a hormone that acts on the mammary glands of lactating females to stimulate the let-down of milk. Oxytocin can be acquired from a veterinarian.
2.2 Administer the anesthetic IM. Our laboratory uses IACUC approved substances, including either midazolam or a mixture of butorphanol, azaperone and medetomadine (BAM) in the following doses:
■ midazolam 1-2 mg/kg
■ butorphanol 0.45 mg/kg
■ azaperone 0.25 mg/kg
■ medetomadine 0.07 mg/kg
2.2.1 One dose of the BAM anesthetic mixture will sedate a muntjac for approximately 20-30 minutes, which provides ample time for complete milk collection. One dose of the anesthetic midazolam will sedate a muntjac for approximately 10-15 minutes, which provides adequate time for milk collection; however, the anesthetic is metabolized quickly and the animal may attempt to escape at the end of that time period.
2.2.2 If milking more than one doe in a session, it is necessary to complete the procedure on one animal before anesthetizing another.
2.3 Apply a small amount of lubricant to the corner of each eye and spread to prevent the muntjac's eyes from drying out while under anesthesia.
3. Milk Collection
Note: The milk collection is most easily performed with two researchers; one researcher to hold the anesthetized muntjac in the lap while manually expressing the milk (Referred to as R1), and a second researcher to keep the muntjac's head in a comfortable and safe position and to collect the milk (R2).
3.1 Depending upon the anesthetic used, milking should be performed either 1) in their pens to provide a safe place for them should they wake up during the procedure (midazolam-induced) or 2) in a bedding-free anteroom to maintain cleanliness (BAM-induced).
3.2 R1 and R2 should be equipped with ethanol-soaked gauze and several 15 ml conical polypropylene tubes to hold the milk.
3.3 As the oxytocin begins to take effect, milk letdown will become visible in the muntjac's mammary area. R1 cleans his/her gloved hands and fingers, as well as the entire udder and each individual teat of the animal with the ethanol-soaked gauze. R2 will hold the muntjac's head in a safe and comfortable position to allow ease of breathing and help prevent rumen regurgitation. R2 will also hold the collection tube close to the teat being milked without touching it.
3.4 R1 expresses milk from one teat by using the thumb and forefinger to gently squeeze the mammary tissue in an upward motion until the teat is engorged with milk, and gently roll the milk out of the teat and into the tube that R2 is holding. The milk comes out in a stream, so it is important for R2 to have the collection tube positioned so that little to no milk will be wasted.
3.5 The muntjac's udder is divided into quadrants. R1 will continue to express milk from each quadrant until the milk no longer flows easily. If milk cannot be expressed from one teat, R1 will express all other teats before returning to the first. It is both acceptable and useful to come back to and continually express milk from the same teat should it produce more milk than another.
4. Anesthetic reversal in the doe
4.1 It is common for midazolam-induced doe to begin to wake up during the milking process. In most cases, the doe will attempt to escape before all the milk has been expressed. Minor restraint is used in an effort to increase the milk yield; however the safety and comfort of both the researchers and the doe are equally important.
4.2 In the BAM-induced doe, atipamezol (2.5 mg/mg medetomadine) subcutaneously is administered for rapid reversal.
4.3 In both cases, it is necessary to maintain close observation of the doe until she is able to walk on her own without stumbling or falling, and is able to eat and drink without fear of aspiration or choking. No ill effects have been noted with respect to the fawn's ability to nurse after milk collection.
Representative Results
Mouse
From our experiments, we have determined that it is possible to collect approximately 100-400μl) of milk from one laboratory mouse, dependent on several variables. These variables include 1) the amount of time set aside for collection, 2) the dose of oxytocin administered, 3) how many pups the dam is currently nursing and 4) the amount of time post parturition. Our studies have shown that the highest yield is obtained when the dams are separated from their offspring for at least two hours, and at least 45 minutes to an hour is set aside per mouse for milking. Maximum milk volumes were harvested 8-12 days post parturition using 2 IU/kg of oxytocin. Our results are in accordance with other studies where litter size was positively correlated with milk yield (9). (Table 1)
Muntjac
Although the amount of milk harvested from the muntjac was variable from day to day, we were able to collect 5 to 30 ml milk/dam/session, depending on the anesthesia used and/or time post parturition. Midazolam-induced doe remained under anesthesia for as little as ten minutes, allowing for smaller volume collections (typically 5-30 ml), whereas BAM-induced doe sustained a longer period of anesthetic effect (20-30 minutes), allowing complete milk expression (15-130 ml). Milk collections were discontinued when milk was no longer being produced in sufficient amounts by the doe, and the corresponding fawn was being nourished on a diet of mostly hay forage.
Discussion
Mouse
There are several factors to take into consideration when collecting milk from a mouse, including 1) the amount of time set aside for collection, 2) the dose of oxytocin administered, 3) how many pups the dam is currently nursing and 4) the amount of time that has passed since parturition at the time of collection. Using previous studies as guidelines, we set out to optimize conditions for milk yield.
Previous research has directly correlated milk yield with litter size (9). Unfortunately, litter size is a variable that cannot be controlled. However, the time of milking post parturition can be controlled. According to “The Laboratory Mouse” (9) maximum milk secretion is at 8-10 days post parturition. Our experience is in congruence with this statement.
It has been shown that, while oxytocin is not required for reproduction in mice, it is essential for milk production (10). Our experiments demonstrate a positive correlation between the amount of oxytocin administered and the yield of milk collected (Table 1). Oxytocin doses ranging from 0.1 IU/kg (9) to 4 IU/kg (6) have been reported for collection of milk from rodents. We began our study by administering the lowest dose suggested, 0.1 IU/kg, but found the volume of milk collected to be inadequate (too small to measure.) Upon increasing the oxytocin dose to 2 IU/kg (1), milk yield was considerably higher at 120 μl. Because we wanted to use the lowest dosage of oxytocin to achieve optimal milk collection, we did not administer doses higher than 2 IU/kg.
Milk yield is also dependent upon the amount of time set aside for collection. We have determined that a minimum of three hours is necessary for optimal collection—two hours for the pups to be separated from the dam and one hour for material preparation and collection. Collecting milk from several dams in one session will decrease the overall time necessary to harvest a specific volume of milk i.e., two hours to separate pups, 30 minutes to setup materials and 20-30 minutes to collect milk from each dam. Each dam must be monitored until they are awake before being placed back with their pups. We have noted that the more time set aside for milking leads to higher milk yields, because the mouse can be milked until she regains consciousness.
Instances do arise with animal models that will affect milk yield. One common issue is the presence of blood or exudate in the milk of older dams that have been bred repeatedly and have large litters, or in dams that are nursing their first litter. Should this occur, we suggest discontinuing milk collection from that particular teat. It is advisable to switch pipet tips at this time, and discard any exudate or blood.
Researchers carrying out this method should also be aware that, as is often the case when using animal models, results vary (Table 1). If a less than optimal amount of milk is collected in one session, it is preferable to repeat the procedure at a later time (e.g. 24-48 hours) rather than alter the experimental conditions to force milk release.
Muntjac
The volume of milk collected from Reeves’ muntjac dams was dependent upon the anesthetic used. Our initial anesthetic of choice was midazolam. Midazolam provided sufficient sedation to allow restraint for short periods of time—i.e. if R1 had a calm disposition and could keep the muntjac calm, R2 could take over the milking to increase the yield before the muntjac would awaken— the safety and comfort of both animal and handler were of utmost importance and we abandoned the milking effort if we felt either was compromised. We discovered that muntjac doe quickly become tolerant to midazolam and that higher doses would be necessary after repeated exposure, making milk collection twice weekly increasingly difficult.
The shortage of midazolam, coupled with a building tolerance, led us to research an alternative anesthestetic. A mixture of Butorphanol, Azaperone and Medetomadine (BAM) is effectively used for the immobilization of white tail deer (11). In our hands, the BAM cocktail provided sufficient anesthesia to allow collection of acceptable volumes of milk in a safe manner, with less drug tolerance after repeated use, and with better control in reversing the effects of the anesthetic.
Some muntjac dams suffered from sensitive udders and/or were first-time mothers. These muntjac developed bruising on the teats during the milking process, and the milk flow slowed or stopped completely, prompting us to discontinue the ongoing session. Should this occur, one should use a more gentle milking technique in an attempt to prevent discomfort or bruising in the teats.
Milk production appeared to wax and wane on several occasions. Low milk harvests may be caused by 1) the fawns having nursed just prior to collections or 2) a decline in milk production due to the natural process of fawn weaning.
While it can be a trying process to obtain a sizeable volume of milk from small animal models, our work illustrates that it is indeed a possibility for both mouse and muntjac models; neither requiring extensive skill nor deep pockets, simply perseverance.
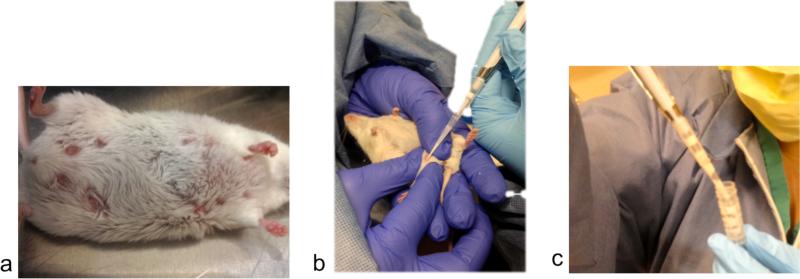
Figure 1.
a.) The dam's teats become engorged with milk upon oxytocin injection. b.)
Collection of milk from an anesthetized dam using a P-200 pipetman. c.) Ejection of milk into a 1.2 ml cryovial.
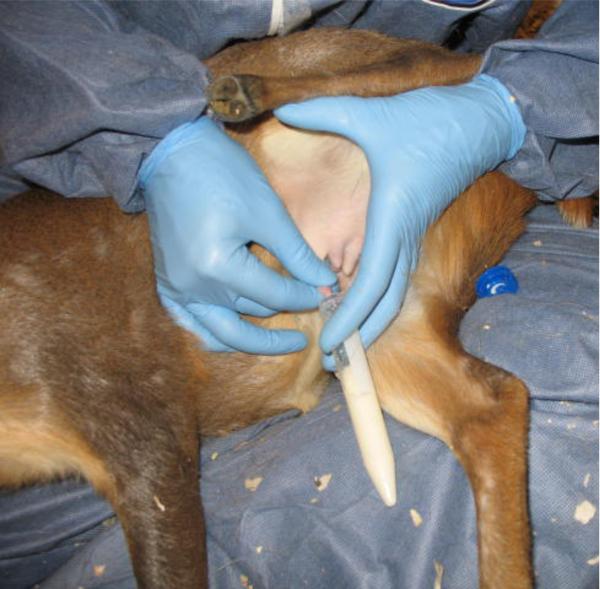
Figure 2.
Muntjac milk is collected into a 15 ml conical tube.
Table 2.
Milk collection start and finish dates for Reeves' muntjac
| Muntjac # | Date started | Date Finished | Total days |
|---|---|---|---|
| 19 | 06.10.08 | 11.06.08 | 150 |
| 16 | 07.02.08 | 11.06.08 | 128 |
| 11 | 05.05.09 | 06.25.09 | 52 |
| 19 | 04.01.10 | 05.20.10 | 50 |
| 44 | 04.06.10 | 04.23.10 | 18 |
| 45 | 04.06.10 | 05.20.10 | 44 |
| 77 | 01.05.12 | 04.19.12 | 106 |
| 75 | 01.03.12 | 04.19.12 | 108 |
| 73 | 01.03.12 | 04.19.12 | 108 |
| 76 | 02.08.12 | 04.19.12 | 71 |
| 111 | 09.13.12 | 10.30.12 | 48 |
| Average | 80.27 |
Acknowledgments
These experiments were funded by the NIH grant #RAI093634A. We express immense gratitude to Jenny Powers, DVM, PhD, for her assistance in muntjac milking.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Deuling S. Muntiacus reevesi. Animal Diversity Web. 2004 [Google Scholar]
- 2.Pei K, Taber R, et al. Breeding cycle of the Formosan Reeves muntjac (Muntiacus reevesi micrurus) in northern Taiwan, Republic of China. Mammalia. 1995;59(2) [Google Scholar]
- 3.Browning SR, Mason GL, Seward T, et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol. 2004;78(23):13345–50. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van de perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21(24):3374–6. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 5.Maden Charlotte. Genetically modified mice produce human milk protein. BioNews. Issue 511 [Google Scholar]
- 6.Rodgers CT. Practical aspects of milk collection in the rat. Lab Anim. 1995;29(4):450–5. doi: 10.1258/002367795780739980. [DOI] [PubMed] [Google Scholar]
- 7.Depeters EJ, Hovey RC. Methods for collecting milk from mice. J Mammary Gland Biol Neoplasia. 2009;14(4):397–400. doi: 10.1007/s10911-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight CH, Maltz E, et al. Milk yield and composition in mice: effects of litter size and lactation number. Comp Biochem Physiol. 1986;84(1):127–33. doi: 10.1016/0300-9629(86)90054-x. [DOI] [PubMed] [Google Scholar]
- 9.Hedrich Hans J. Gillian R. Bullock. The laboratory mouse. Elsevier Academic Press; Amsterdam: 2004. Print. [Google Scholar]
- 10.Nishimori K, Young LJ, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93(21):11699–704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller BF, Osborn DA, et al. Butorphanol-azaperone-medetomidine for immobilization of captive white-tailed deer. J Wildl Dis. 2009;45(2):457–67. doi: 10.7589/0090-3558-45.2.457. [DOI] [PubMed] [Google Scholar]