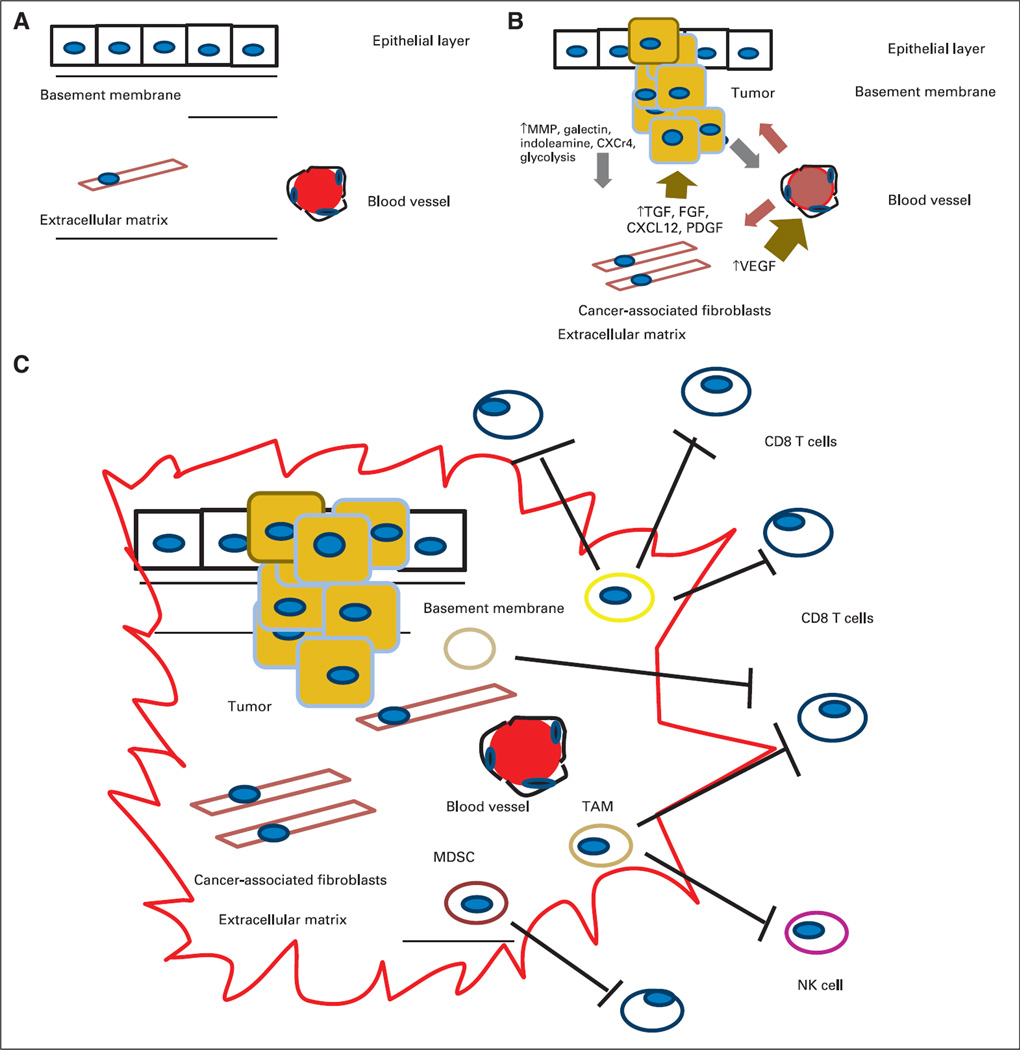
Fig 1.
(A) Normal relationship between the epithelial layer, basement membrane, and extracellular matrix (ECM). (B) Interaction between the tumor initiation process and its relationship to the stroma. A pathologic hallmark of pancreatic cancer is the development of an abundant inflammatory response. The inflammatory environment consists of activated stellate cells, ECM proteins, and fibroblasts. These cancer-associated fibroblasts are thought to secrete factors such as transforming growth factor beta (TGF-β). This subsequently leads to the production of ECM components, including collagens, fibroblast growth factor, C-X-C chemokine ligand 12 (CXCL12), proteoglycans, and hyaluronic acid. These in turn secrete tumor-promoting factors that contribute to the tumor’s invasion through the basement membrane via proteolytic enzymes, including matrix metalloproteinases. There are also increases in angiogenic factors such as vascular endothelial growth factor (VEGF) that lead to the development of new blood vessels and changes in vascular permeability that lead to the release of additional ECM-modulating events. (C) Tumor initiation-stroma interaction. Mutated K-ras is thought to be the driver of a cancer-associated inflammation program that leads to predominance and infiltration of immunosuppressive immune cells into the tumor stroma at the expense of effector T cells. Tumors harbor the ability to increase the number regulatory T cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) and to upregulate molecules such as C-X-C chemokine receptor type 12. Ultimately, they produce a local immunosuppressive environment ideal for tumor growth. CXCR4, C-X-C chemokine receptor type 4; NK, natural killer (cell); PDGF, platelet-derived growth factor; PGF, placental growth factor.