Figure 3.
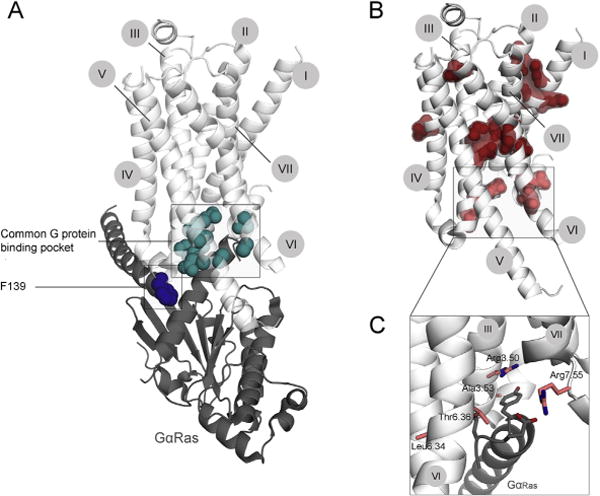
Mutations alter the ability of the receptor to transduce the signal. A. Receptor residues essential for GPCR-G protein interaction are shown on the structure of active β2AR in complex with the Gs protein (Ras-like domain of Gα-subunit is shown in dark gray). They include several residues within the cytoplasmic cavity (turquois), and the Phe139, which is engaged in a hydrophobic interaction with a number of residues of Gα Ras-like domain. Mutations of any of these residues can be expected to alter GPCR-G protein coupling and thus transduction ability. B. Mutations, altering maximum response along with normal cell surface receptor expression, are shown on the active structure of β2AR. Depending on the localization of the mutation, we propose two different mechanisms of its action. Residues located within the center or far from the cytoplasmic site of the receptor (dark red) are expected to alter overall receptor conformational equilibrium; residues located at or within close proximity to the G protein-binding interface (light red) are expected to directly alter the GPCR-G protein interaction. For a detailed view of those residues (C) the perspective was changed slightly for better visualization. The numbers of trans-membrane helices I–VII are indicated in gray circles.
