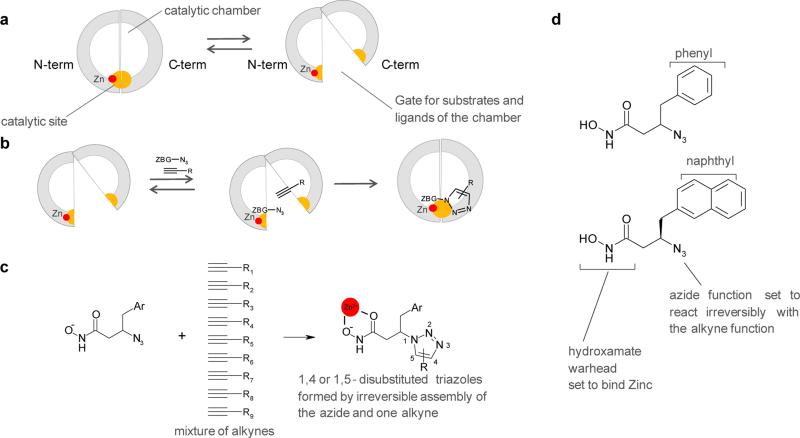
Figure 1. Use of TGS to design catalytic site inhibtors of IDE.
(a) Schematic view of IDE showing the catalytic site in yellow formed inside the catalytic chamber by the N- and C-terminal domains. The equilibrium between closed and open conformations is shown. (b) Principle of kinetic TGS: an azide-bearing hydroxamate warhead (ZBG-N3) and an alkyne are shown bound to the enzyme and reacting irreversibly to form a triazole. (c) IDE chooses reagents amongst a mixture of alkynes (d) The two azide-bearing hydroxamate warheads used in the experiment.