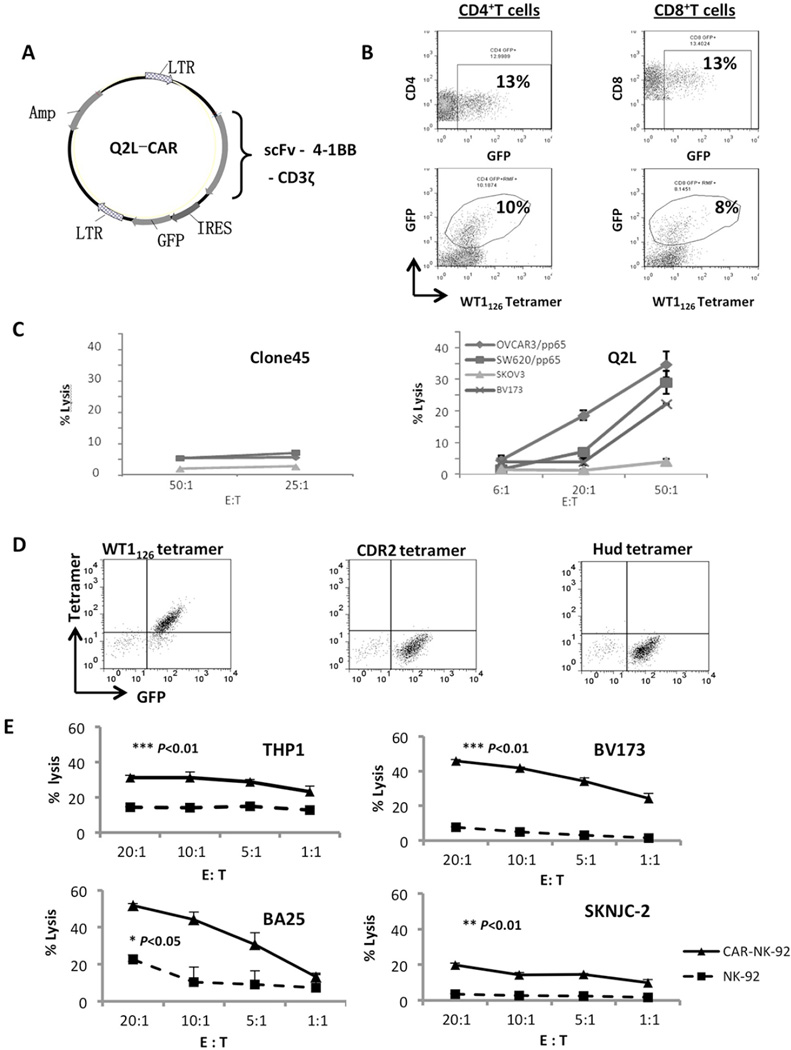
Figure 5. Chimeric antigen receptor expressing human lymphocytes specific for HLA-A2-WT1126.
(a) Schematic diagram of the CAR construct. The scFv sequence was cloned into the CAR gene and further cloned into a murine stem cell virus-based vector, which contained an internal ribosome entry site (IRES)-green fluorescence protein (GFP) sequence along with ampicillin-resistance. The resulting CAR was composed of the leader sequence, scFv and hinge region on the extracellular surface, a CD8α transmembrane domain, along with 4-1BB and the CD3ζ chain. (b) Transduced T cells derived from a single healthy donor. Both CD4 and CD8 T cells were genetically modified. CAR modified T cells were stained with HLA-A2-WT1126 tetramer, anti-CD4, or anti-CD8 and analyzed by flow cytometry. (c) Specific cytotoxicity of Clone45-CAR (left) or Q2L-CAR (right) T cells against the tumor cell lines by chromium release assay. Samples were prepared in triplicate and values are shown as mean ± SE. Experiment was repeated twice with similar results. (d) CAR NK-92 cells were stained with PE conjugated HLA-A2/WT1126 tetramer (right) and two isotype controls: HLA-A2/Hud tetramer and HLA-A2/CDR2 tetramer. (e) Specific cytotoxicity of Q2L-CAR NK-92 (solid line) and mock (dashed line) cells against the tumor cell lines by chromium release assay. Samples were prepared in triplicate, and values are shown as mean ± SD. Experiment was repeated twice with similar results. The P values of the difference between CAR and mock groups were analyzed by log-rank Mantel-Cox test, with treatment groups showing similar variance.