Table 1.
Catalytic activity of wild-type myoglobin (Mb) and its variants in the oxidation of benzyl azide to benzaldehyde.[a]
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Catalyst | [catalyst] / mM |
pH | Conv.[b] | TON[c] |
| 1 | Hemin | 0.02 | 8.0 | 19% | 95 |
| 2 | Hemin + imidazole (1 mM) |
0.02 | 8.0 | 22% | 110 |
| 3 | WT Mb | 0.001 | 8.0 | 18% | 1,650 |
| 4 | Mb(L29A) | 0.001 | 8.0 | 19% | 1,630 |
| 5 | Mb(F43W) | 0.001 | 8.0 | 23% | 2,190 |
| 6 | Mb(F43V) | 0.001 | 8.0 | 32% | 2,980 |
| 7 | Mb(H64V) | 0.001 | 8.0 | 41% | 3,500 |
| 8 | Mb(V68A) | 0.001 | 8.0 | 15% | 1,420 |
| 9 | Mb(V68F) | 0.001 | 8.0 | 6% | 580 |
| 10 | Mb(L29A,H64V) | 0.001 | 8.0 | 36% | 3,110 |
| 11 | Mb(F43V,V68A) | 0.001 | 8.0 | 42% | 3,380 |
| 13 | Mb(H64V,V68A) | 0.001 | 8.0 | 49% | 3,740 |
| 14 | Mb(H64V,V68A) | 0.001 | 7.0 | 77% | 6,340 |
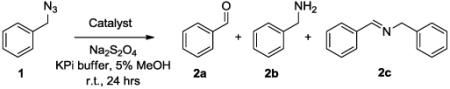
Reactions (400 μL) were conducted under anaerobic conditions with 10 mM BnN3 and 10 mM Na2S2O4 for 24 hours at room temperature.
Product yield based on conversion of initial 1a to 2a as determined by gas chromatography.
= nmol aldehyde / nmol catalyst. Errors in reported values are within ± 10%.
