Table 1.
Catalytic Asymmetric Aldol Reaction of Azlactone 6a and aldehyde 7aa
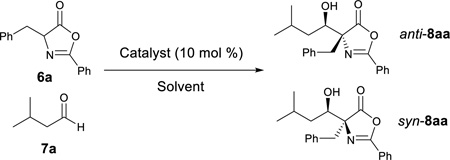 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Temp(°C) | Time | Conv. (%)c | ee (%)c, d | antilsync |
| 1 | 1 | CHCI3 (2 M) | −20 | 15 h | >95 | 29/22 | 43.5/56.5 |
| 2 | 2 | CHCI3 (2 M) | −20 | 15 h | 93 | 43/12 | 40.5/59.5 |
| 3 | 3a | CHCI3 (2 M) | −20 | 15 h | >95 | 57/25 | 81/19 |
| 4 | 3b | CHCI3 (2 M) | −20 | 15 h | >95 | 51/19 | 73/27 |
| 5 | 3c | CHCI3 (2 M) | −20 | 15 h | 92 | −7/18 | 71/29 |
| 6 | 3d | CHCI3 (2 M) | −20 | 15 h | >95 | 75/13 | 88/12 |
| 7 | 3e | CHCI3 (2 M) | −20 | 15 h | >95 | −73/−16 | 90/10 |
| 8 | 4 | CHCI3 (2 M) | −20 | 15 h | 91 | −64/−6 | 82/18 |
| 9 | 5 | CHCI3 (2 M) | −20 | 15 h | >95 | −71/22 | 66/34 |
| 10 | 3d | CHCI3 (0.5 M) | −20 | 34 h | 95 | 86/−11 | 91/9 |
| 11 | 3d | CH2CI2 (0.5 M) | −20 | 34 h | 93 | 86/−39 | 93.5/6.5 |
| 12 | 3d | PhCH3 (0.5 M) | −20 | 34 h | 80 | 48/−28 | 80/20 |
| 13 | 3d | THF (0.5 M) | −20 | 34 h | >95 | 50/−28 | 79.5/20.5 |
| 14 | 3d | Et2O (0.5 M) | −20 | 34 h | >95 | 53/−28 | 83/17 |
| 15 | 3d | CH3CN (0.5 M) | −20 | 34 h | >95 | 72/−18 | 88/12 |
| 16e, f | 3d | CH2CI2 (0.1 M) | −50 | 88 h | >95 (92)b | 94/ND | 97.5/2.5 |
Reactions were carried out with 0.1 mmol of 6a and 0.15 mmol of 7a.
Isolated yield.
Determined by chiral HPLC analysis.
ee (antilsyn).
10 mg of 4Å molecular sieves were added.
15 mol% 3d
