Table 1.
Solvent screen for cyclopropenimine-catalyzed enantioselective Michael reaction.a
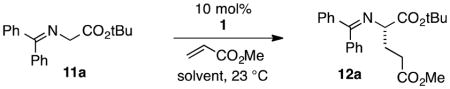
| |||||
|---|---|---|---|---|---|
| entry | solvent | ε | time(h) | yield (%) | ee (%) |
| 1 | 1,4-dioxane | 2.3 | 8 | 95 | 98 |
| 2 | PhMe | 2.4 | 5 | 95 | 99 |
| 3 | NEt3 | 2.4 | 6 | 95 | 99 |
| 4 | Et2O | 4.3 | 2 | 95 | 98 |
| 5 | EtOAc | 6.0 | 2 | 86 | 98 |
| 6 | THF | 7.5 | 24 | 95 | 89 |
| 7 | CH2Cl2 | 9.1 | 10 | 95 | 86 |
| 8 | 1,2-F2-C6H4 | 14.3 | 4 | 95 | 89 |
| 9 | n-BuOH | 17.5 | 24 | 45 | 55 |
| 10 | acetone | 21 | 1.5 | 95 | 80 |
Conversion determined by 1H NMR based on Bn2O standard. Enantiomeric excesses (ee) were determined by chiral HPLC. ε: dielectric constant.
