Fig. 2.
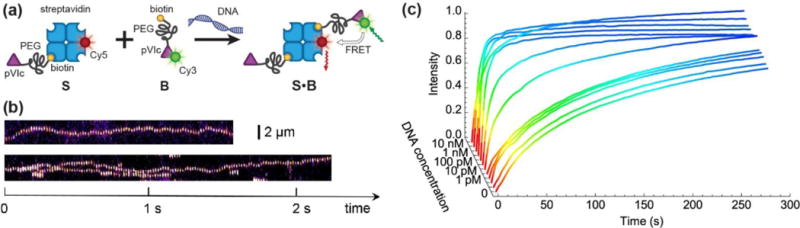
Speeding up bimolecular association by DNA. (a) A schematic of the proof-of-concept biotin-streptavidin system. (b) Single-molecule fluorescence imaging confirm that functionalization of binding partners with pVIc peptide renders S and B able to 1D slide along DNA. The results are presented as kymographs: top trace shows the sliding of binding partner S, bottom trace shows the sliding of binding partner B, labelled with streptavidin-Cy5 for the ease of detection in view of the signal-to-noise ratio. (c) The formation of complex S•B is monitored in time using various concentrations of 2686-bp long DNA in solution. The time evolution of product concentration can be approximated by exponential growth , where τ is the observed characteristic reaction time.
