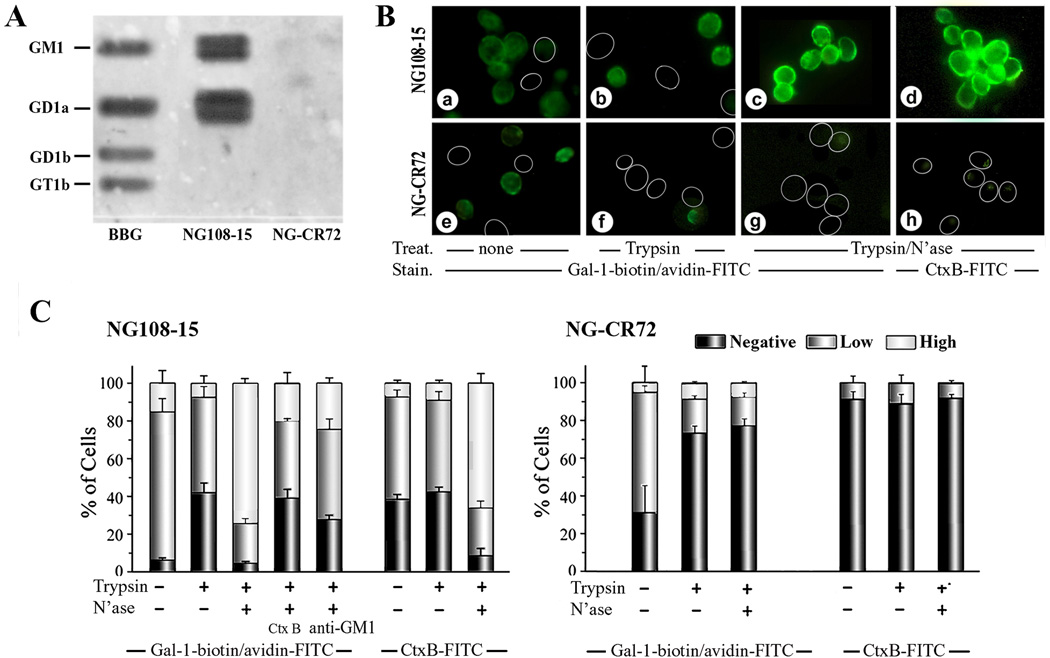
Figure 1.
Binding of Gal-1 to ganglioside GM1 and to cell surfaces. (A) Documentation of Gal-1 binding to gangliosides after HPTLC. Lipids extracted from NG108-15 and NG-CR72 cells, together with bovine brain gangliosides (BBG), were separated by HPTLC. Following treatment with N’ase that converted di- and trisialylated gangliosides to GM1, the plate was successively probed with biotinylated Gal-1 and avidin-HRP to demonstrate interaction of Gal-1 with GM1; this also reveals the presence of a-series gangliotetraoses in NG108-15 but not NG-CR72 cells. (B) Documentation of Gal-1 binding to intact cells. NG108-15 (a–d) and NG-CR72 (e–h) cells treated with trypsin alone (b, f) or trypsin/N’ase (c, d, g,h) were stained with Gal-1-biotin/avidin-FITC (a–c, e–g) and with Ctx-B-FITC (d, h). Circles = unstained cells. (C) Extent of Gal-1 binding in semiquantiative categories. In addition to results in panel B, Ctx-B and anti-GM1 were tested as inhibitors of Gal-1 binding to NG108-15 cells.