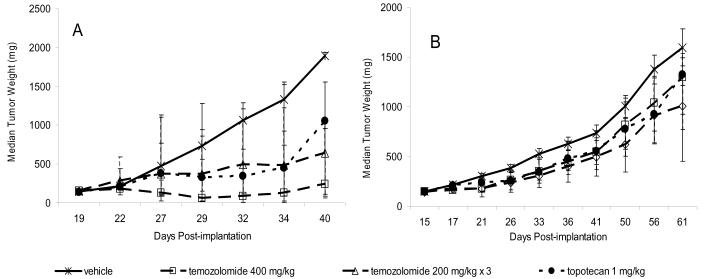
Figure 2.
Activity of temozolomide and topotecan in human tumor xenografts. A) A375 melanoma xenografts. B) Colo 829 melanoma xenografts. Cells of both lines were implanted subcutaneously in female athymic nude (nu/nu NCr) mice (Animal Production Program, NCI-Frederick). Treatment was initiated when the tumors reached 150 mg. Temozolomide was administered by oral gavage as a single dose of 400 mg/kg or as three 200 mg/kg doses given 4 days apart (temozolomide 200 mg/kg × 3) (n=10 mice/dose group). Topotecan was administered intraperitoneally at 1 mg/kg 5 days per week for 2 weeks (n=10). The vehicle control group (n=20) was treated with three doses of saline given 4 days apart. Individual tumor weights were calculated as weight in mg = [length × width2]/2. Data are plotted as median tumor weights +/− 95% confidence intervals.