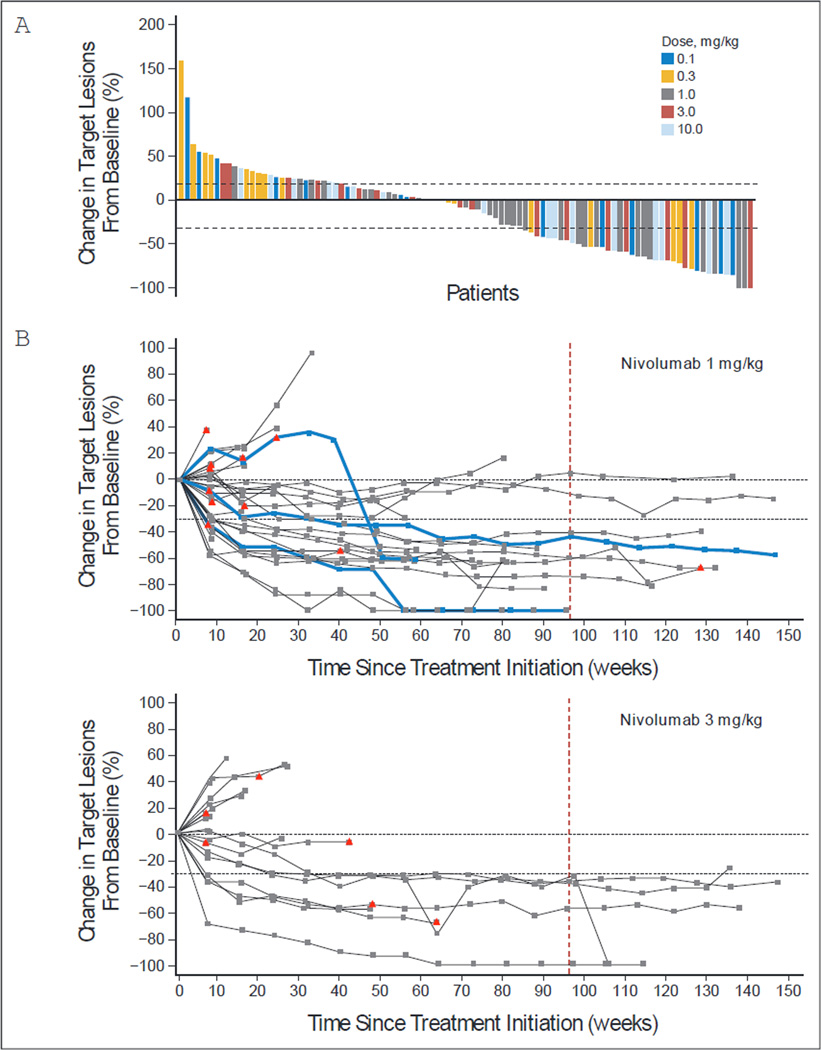
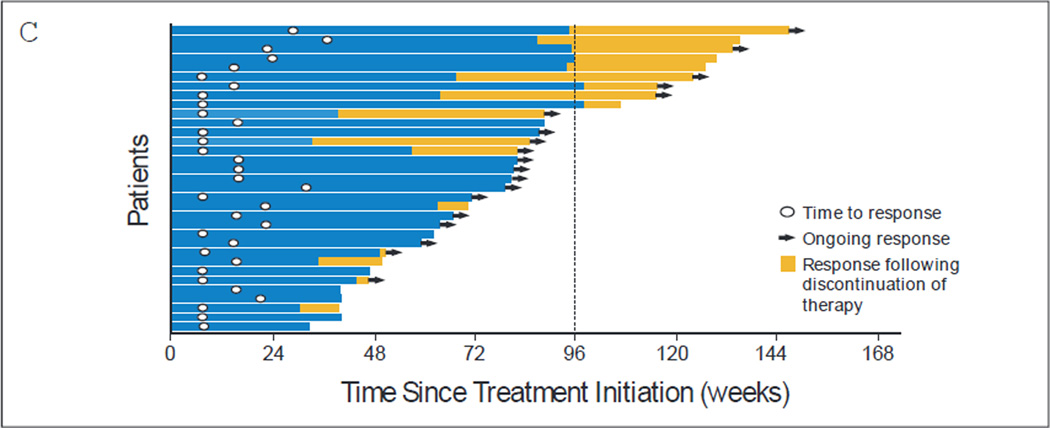
Fig 2.
Characteristics of tumor regression in patients with melanoma receiving nivolumab therapy. (A) Maximum reduction or minimum increase in the sum of target lesion measurements compared with baseline in all treated patients with tumor measurements during treatment (n = 97). Responses were observed at all dose levels. Horizontal line at +20% indicates the threshold for defining progressive disease according to RECIST. Horizontal line at −30% indicates threshold for defining objective response (partial tumor regression) in the absence of new lesions or nontarget disease progression according to RECIST. (B) Response kinetics in patients receiving nivolumab at 1 mg/kg (n = 31) or 3 mg/kg (n = 17). Baseline tumor measurements are standardized to zero. Tumor burden is measured as the sum of the longest diameters of target lesions. Triangles indicate first occurrence of a new lesion. Vertical line at 96 weeks indicates the protocol-defined maximum duration of continuous nivolumab therapy. Horizontal line at −30% marks the threshold for defining objective response (partial tumor regression) according to RECIST. Blue curves indicate three unconventional immune-related response patterns in the 1 mg/kg dose cohort that did not meet RECIST criteria (eg, persistent reduction in target lesions in the presence of new lesions or regression following initial progression). Objective responses, unconventional responses, and stable disease persisted following treatment discontinuation in some patients. (C) Durability of tumor regressions in patients with melanoma who had objective responses to nivolumab therapy according to conventional RECIST criteria. Among 107 patients, 33 (31 %) responded, including six (35%) of 17 who received nivolumab at 0.1 mg/kg, five (28%) of 18 at 0.3 mg/kg, 11 (31 %) of 35 at 1 mg/kg, seven (41 %) of 17 at 3 mg/kg, and four (20%) of 20 at 10 mg/kg. Blue bars indicate the time to and duration of response while on treatment; gold bars indicate response duration after treatment discontinuation; open circles indicate first evidence of objective response; arrows indicate ongoing response at time of analysis. Vertical line at 96 weeks indicates maximum planned duration of continuous nivolumab therapy. Reasons for treatment discontinuation with ongoing response included investigator-assessed complete response (n = 2), attainment of maximum treatment duration (n = 5), adverse events (n = 6), and other (eg, withdrew consent or investigator decision [n = 4]).