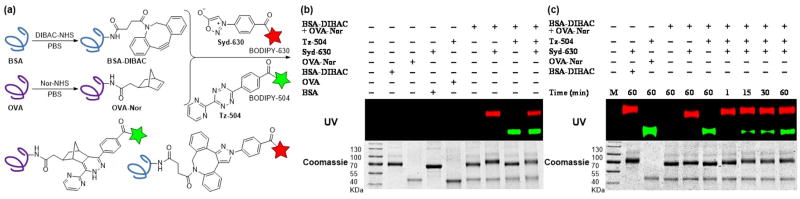
Fig. 3.
Modifications of protein surfaces via bioorthogonal cycloadditions. (a) Dibenzoazacyclooctyne (DIBAC) and 5-norbornene-2-acetic acid (Nor) were appended to BSA and OVA (20 mg/mL in PBS) respectively, via NHS ester-amine coupling conditions (20 mM labeling reagent). The labeled proteins BSA-DIBAC and OVA-Nor (2 mg/mL) simultaneously react with sydnone-BODIPY (Syd-630) and tetrazine-BODIPY (Tz-504). (b) Gel analysis of BSA functionalized with DIBAC and OVA functionalized with Nor incubated for 1 h with either Syd-630, Tz-504, both reagents simultaneously, or no reagent (−). (c) Gel analysis of DIBAC-modified BSA and Nor-modified OVA with both Syd-630 and Tz-504 simultaneously for 1–60 min or no reagent (−). The protein loading on the gels was assessed with Coomassie stain.