Figure 3.
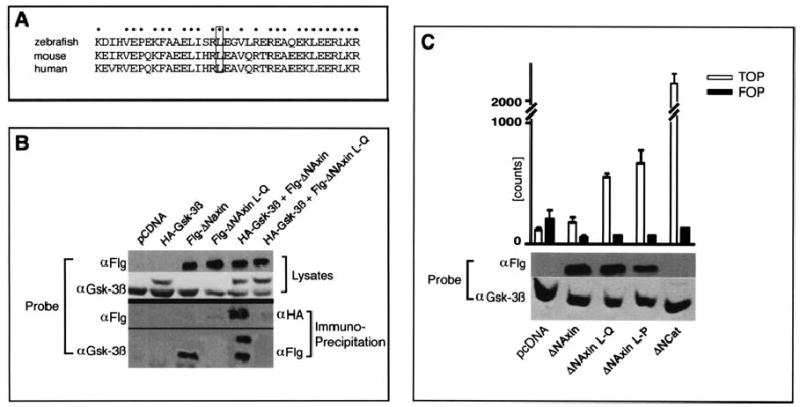
The mbl mutation a bolishes binding of Axin to Gsk3. (A) Comparison of the amino-acid sequence around the L→Q mutation site within the Gsk3 binding domain of Mbl/Axin1 between zebrafish, mouse, and human shows a high degree of conservation. (B) Assessment of binding of Gsk3 β to a wild-type Axin construct (FlgΔN-Axin) and to an Axin construct incorporating the L→Q amino-acid exchange (FlgΔN-Axin L–Q). Wild-type and/or mutant (L–Q) FlgΔN-Axin and HA-Gsk3β were immunoprecipitated and immunoblotted with α-Gsk-3β and α-Flg antibodies. The L–Q mutation prevents both endogenous Gsk-3-β and HA-Gsk-3β association with the Axin protein. The ΔN variant of Axin (amino acids 351–965) was used in preference to full length Axin because of its greater solubility in vitro (Smalley et al. 1999). (C) Assessment of TCF-dependent transcription in the presence of Flg-ΔNAxin and Flg-ΔNAxin L–Q. Expression of Flg-ΔNAxin L–Q activates TCF-dependent transcription indicating that it can act as a dominant active protein while wild-type Flg-ΔNAxin has little or no effect compared to control (pcDNA). The dominant effect of Flg-ΔNAxin L–Q is comparable to the previously described L–P mutation at the same position (lane4; Smalley et al. 1999). Expression of transfected Axin and endogenous Gsk3β (control) is shown by immunoblot analysis. Lane 5 shows transcription following transfection with a constitutively active form of beta;-catenin. Counts are luciferase reporter assay readouts from TOP-FLASH plasmid (TCF-binding motif) and FOP-FLASH plasmid (mutant motif as control) that have been adjusted for transfection efficiency as described previously (Smalley et al. 1999).
