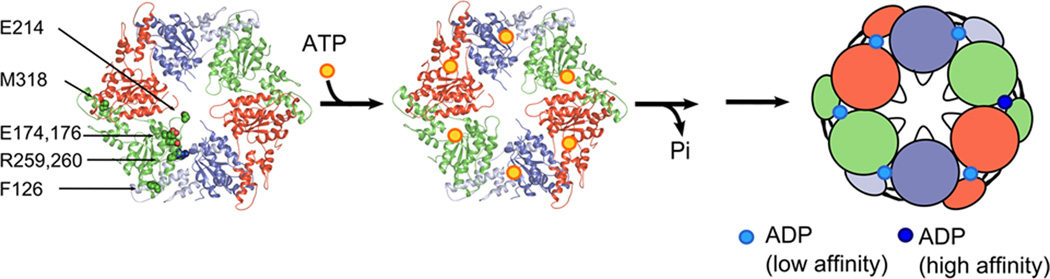
Figure 6. Model for Vps4 action.
The MsVpsΔMIT pseudohexamer is represented as a cartoon, with identical protomers shown in the same color. (a) The nucleotide free pseudohexamer, (b) the pseudohexamer may bind six ATP (yellow sphere), which are likely required to assemble the catalytic active ring structure, (c) ATP hydrolysis generates intermediate states, which leads at one point of the cycle to an intermediate conformation that binds one ADP with high affinity and 5 with low affinity. The position of the arginine fingers, the glutamate (174, 176) V interface residues, the pore loop glutamate (214) and phenylalanine 126 are indicated. These residues are required for ATPase activity and most likely participate in the transfer of ATP hydrolysis induced conformational changes of two opposing monomers to their neighbors, thereby powering the disassembly of ESCRT-III substrate complexes via the central pore47,52.