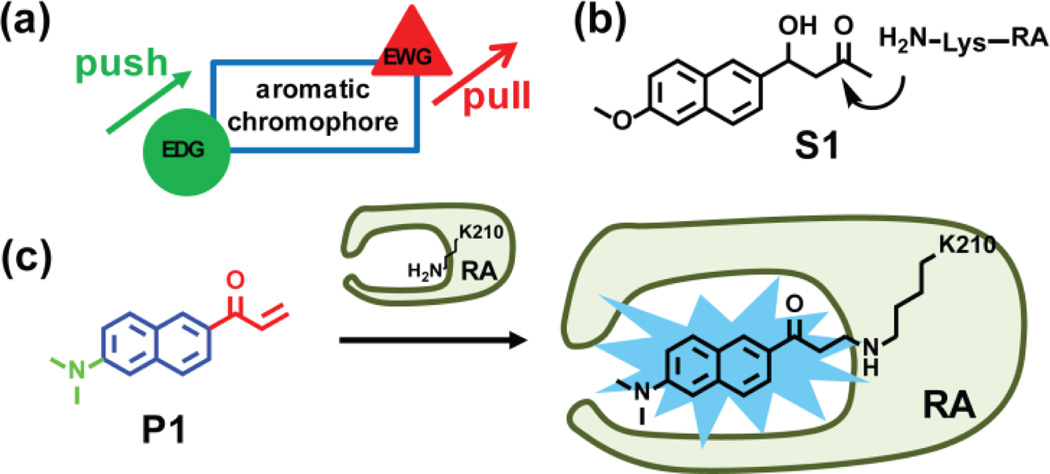
Figure 2.
Structure-based design of a fluorogenic probe for folded and functional RA. (a) Schematic of a push-pull environmentally-sensitive fluorophore. EWG = electron-withdrawing group. EDG = electron-donating group. (b) Structure of the retroaldol substrate S1 utilized by the de novo designed RA enzyme.29 RA catalyzes a retroaldol reaction using the pKa-perturbed lysine-210 ε-amine side chain that forms a Schiff base with S1. (c) P1 is a push-pull environmentally-sensitive fluorophore featuring a reactive vinyl ketone (in red) that also serves as an electron-withdrawing group. The dimethyl amino group (in green) serves as an electron-donating group. P1 covalently modifies the pKa-perturbed lysine-210 residue of RA through 1,4-conjugate addition, rendering the RA-P1 covalent conjugate fluorescent.