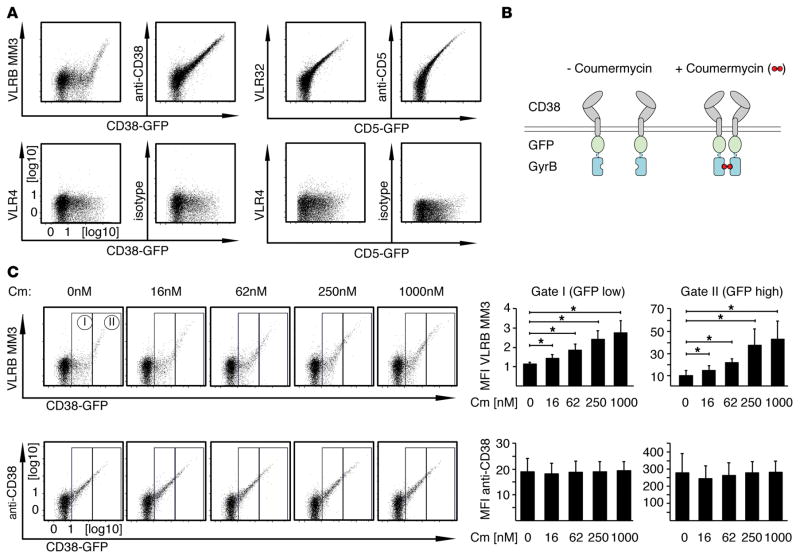
Figure 5. Dimerization of CD38 increases recognition by VLRB MM3.
(A) BJAB cells were transiently transfected with the indicated CD38-EGFP or CD5-EGFP fusion constructs and stained with VLRB MM3 (specific to CD38), VLR32 (specific for CD5), conventional anti-CD38 or anti-CD5 Abs (top row) or with the negative control VLR4 or isotype-matched conventional Ab (bottom row). Ab reactivity was assessed 48 hours after transfection (gated on propidium iodide–negative cells). Shown is a representative of at least 6 independently performed analyses. Note the apparent threshold-based recognition of CD38 by VLRB MM3. (B) Schematic of CD38-EGFP-GyrB fusion constructs, permitting inducible dimerization following addition of coumermycin. (C) BJAB cells transiently transfected with CD38-EGFP-GyrB constructs were stained with VLRB MM3 or conventional anti-CD38 Abs following incubation with coumermycin for 20 minutes at the indicated concentrations. Median fluorescence intensities for reactivity of VLRB MM3 and anti-CD38 Abs normalized to VLR4 and isotype control Abs, respectively, were assessed for the indicated gates on the basis of EGFP expression levels. Depicted are mean fluorescence intensity values ± SD (n = 4). *P < 0.05, by Student’s t test. Cm, coumermycin; MFI, mean fluorescence intensity; VLRB, variable lymphocyte receptor B.