Abstract
Background and Purpose
We evaluated the response of recurrent glioblastoma to superselective intra-arterial cerebral infusion of bevacizumab using DSC-MRP imaging. We hypothesized that treatment response would be associated with decreased rCBV and rCBF.
Materials and Methods
Patients were accrued for this study from larger ongoing serial Phase I/II trials. A total of 25 patients (14 males, 11 females; median age 55 years) were analyzed. Four distinct ROIs were chosen: 1 – normal appearing white matter in the contralateral side, 2 – the location of highest T1 enhancement in the lesion (max enhancing), 3 – the location of highest rCBV in the lesion (max rCBV), and 4 – non-enhancing T2 hyperintense signal abnormality surrounding the tumor (non-enhancing T2 hyperintensity).
Results
There was a statistically significant median percent change of −32.34% (p = 0.001) in rCBV in areas of max rCBV following intra-arterial bevacizumab therapy. There was also a statistically significant median percent decrease in rCBF of −30.67 (p = 0.001) and −27.25 (p = 0.037) in areas of max rCBV and max tumor enhancement, respectively. Lastly, a trend towards statistical significance for increasing rCBV in non-enhancing T2 hyperintense areas (median percent change 30.04; p = 0.069) was noted.
Conclusions
DSC-MRP demonstrated a significant decrease in tumor perfusion metrics within recurrent glioblastomas in response superselective intra-arterial cerebral infusion of bevacizumab, however these changes did not correlate with TTP or OS.
Introduction
Glioblastoma (GB) is the most common and lethal primary malignancy of the central nervous system. Despite a three-pronged intervention consisting of surgical resection followed by radiation with both concurrent and adjuvant temozolomide chemotherapy, the five-year overall survival rate of patients remains approximately 10%.1
While there is no established standard of care for recurrent GB, Bevacizumab (BV) (Avastin; Genentech/Roche, South San Francisco, California) has emerged as a potential treatment option for recurrent GB. BV is a humanized monoclonal antibody that exerts anti-neoplastic effects by inhibiting the angiogenic effects of vascular endothelial growth factor-A (VEGF-A).2, 3 Our group has utilized superselective intra-arterial cerebral infusion (SIACI) following blood-brain barrier (BBB) disruption to improve BV delivery.4 Recently published studies from our group have showed promising results on the safety and efficacy of using SIACI delivery for BV.5, 6
Although treatment with BV produces a dramatic decrease in MRI contrast enhancement, the degree to which these findings reflect actual anti-tumor effects remains unclear.7 BV reduces vessel permeability, which may contribute to changes in enhancement features and potentially confound the relationship between enhancement and tumor response. Hence, the ability of conventional MRI to determine tumor response, progression, and post-treatment effects is not well established.8 Our group previously reported that 1H-MR spectroscopy (MRS) imaging may be a viable method to determine GB response following SIACI BV to overcome the limitations of conventional MRI. 7
Here we evaluate the potential for using dynamic susceptibility contrast enhanced MR perfusion (DSC-MRP) to determine GB response to SIACI BV. Previous studies have highlighted the utility of using DSC-MRP in assessing tumor response, treatment effectiveness, and clinical outcomes in GB patients.9, 10 Specifically, decreases in tumor relative cerebral blood volume (rCBV) and tumor relative cerebral blood flow (rCBF) are associated with favorable clinical outcome, suggesting that changes in rCBV and rCBF could serve as biomarkers for treatment response.9, 10 We hypothesized that treatment response to SIACI BV is associated with decreased rCBV and rCBF, which will correlate with improved survival outcomes.
Materials and Methods
Subjects
Patients were accrued for this study from larger ongoing serial Phase I/II trials of SIACI BV and retrospectively analyzed with approval from the institutional review board of Weill Cornell Medical College. Inclusion criteria for the Phase I/II SIACI BV trials were: recurrent WHO Grade IV glioma refractory to previous combined radiation treatment and chemotherapy with temozolomide, a Karnofsky Performance Scale (KPS) score of > 60 and < 12 doses of prior intravenous BV treatment. Poorly circumscribed enhancing tumors, multifocal tumors, or leptomeningeal spread of tumors were not exclusion criteria. Recurrent GB was diagnosed using follow-up MRI, RANO criteria for progression11 and clinical evaluation. Patients with (1) an increase in contrast enhancing lesion, (2) increase non-enhancing T2/FLAIR lesion in one or two follow-up scans which showed mass effect, infiltration of the cortical ribbon or lesion location outside of the radiation field, (3) any new lesions or (4) clinical deterioration were diagnosed with recurrent disease. Follow-up MRI were compared with MRI obtained within 48 hours postop to appropriately differentiate tumor recurrence from post-op changes.
Inclusion criteria for the current study included patients from the above Phase I/II trials who received brain DSC-MRP imaging within 1–10 days prior to and 3–5 weeks after SIACI BV. A total of 25 patients (14 males, 11 females; median age 55 years, range 29 to 81 years) met inclusion criteria (Table 1). Seven of the 25 patients (28%) received intravenous BV prior to SIACI BV for a mean of 4.7 cycles (range: 0.5 to 9). All but 2 patients received steroids. Time to progression (TTP) and overall survival (OS) were calculated using the date of surgery for primary GB to date of radiologic progression of disease post SIACI BV and date of death. Date of radiological progression was determined using strict RANO criteria by a board certified diagnostic radiologist with a certificate of added qualification in neuroradiology (AJT – 11 years of experience) and a trained senior neuroradiologist (IK – 20 years of subspecialty experience).11
Table 1.
Patient Demographics
| Pt | Age | Sex | Tumor Location | Previous Cycles of Intravenous BV | Steroids | Dose of SIACI BV* | Tx after SIACI BV | TTP+ (days) | OS+ (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | R frontal | 0 | Y | 14 | Lomustine | 851 | 1255 |
| 2 | 60 | F | R temporal-parietal | 0 | Y | 4 | Irinotecan, IV BV | 750 | 889 |
| 3 | 67 | F | R parieto-occipital | 3.5 | Y | 6 | TMZ, IA cetuximab | 1153 | 1357 |
| 4 | 75 | M | R temporal | 0 | Y | 15 | RT | 435 | 504 |
| 5 | 51 | F | L temporal | 0 | Y | 15 | Erlotinib, carboplatin, lomustine | 560 | - |
| 6 | 43 | M | Brainstem | 6 | N | 8 | IA cetuximab | 273 | 336 |
| 7 | 29 | F | Posterior body and splenium of corpus callosum, R thalamus | 0 | Y | 15 | Irinotecan, IV BV | 730 | 1111 |
| 8 | 52 | F | R temporal | 0 | Y | 13 | TMZ | 340 | 514 |
| 9 | 61 | M | L frontal | 0 | Y | 10 | None | 185 | 340 |
| 10 | 66 | M | L parietotemporal | 9 | Y | 15 | None | - | 918 |
| 11 | 61 | F | L occipital, L splenium | 0 | Y | 15 | Carboplatin | 683 | 956 |
| 12 | 58 | F | R parietal | 0 | Y | 15 | None | 133 | 252 |
| 13 | 56 | M | L insula | 0 | Y | 15 | None | 350 | 449 |
| 14 | 55 | M | High parasagittal R parietal | 0 | Y | 15 | None | 618 | 848 |
| 15 | 70 | M | L temporal | 0 | Y | 15 | None | 1186 | 1277 |
| 16 | 81 | F | L occipital | 0 | N | 15 | None | 581 | - |
| 17 | 68 | M | L temporal | 0 | Y | 15 | None | 708 | 776 |
| 18 | 48 | M | R frontal | 0 | Y | 15 | None | 287 | 460 |
| 19 | 44 | F | L temporal | 4 | Y | 8 | Irinotecan, TMZ, IV BV | 300 | 429 |
| 20 | 44 | F | R basal ganglia, R frontal and corpus callosum | 0 | Y | 6 | TMZ, IV BV | 402 | 589 |
| 21 | 30 | M | L frontal, parietal and basal ganglia | 6 | Y | 10 | None | 733 | 795 |
| 22 | 59 | M | R temporal-parietal | 0.5 | Y | 15 | Surgery, IA cetuximab | 1062 | 1152 |
| 23 | 43 | M | L temporal | 0 | Y | 15 | TMZ, IV BV | 231 | 430 |
| 24 | 53 | M | R basal ganglia, genu of corpus callosum | 4 | Y | 6 | None | 328 | 384 |
| 25 | 31 | F | R parietal, occipital and splenium of corpus callosum | 0 | Y | 15 | Carboplatin | 1750 | - |
IA – Intra-arterial; IV – Intravenous; L – Left; OS – Overall survival; Pt – Patient; R – Right; SIACI BV - Superselective intra-arterial cerebral infusion bevacizumab; TMZ – Temozolomide; TTP – Time to progression; Tx – Treatment
Dose in mg/kg
TTP and OS were calculated using the date of surgery for primary GB to date of radiologic progression of disease post SIACI BV and date of death.
Treatment protocol
We have previously described the technical specifications of SIACI and BV treatment.4–6, 12 Briefly, twenty-five percent Mannitol (1.4M) was infused at 10 ml/120 secs to facilitate transient BBB disruption followed by SIACI BV. Subsequently, the appropriate dose of BV was infused over 15 minutes. However, since the Phase I trial aimed to determine the maximum tolerated dose (MTD) of SIACI BV with analysis of 10 escalating doses (2, 4, 6, 8, 10, 11, 12, 13, 14, and 15 mg/kg), the administered dose varied among patients selected for this study. Mean SIACI BV dose received was 12.4mg/kg (range: 4 to 15mg/kg), with 15 patients (60%) receiving the maximum dose 15mg/kg. After a mean 27 days (SD ± 5 days) of observation, all included patients underwent post-infusion imaging. No additional therapy was initiated before the post-SIACI BV MRI-DSC MRP was completed. Fourteen of 25 patients (56%) underwent various subsequent treatments after SIACI BV that included IA cetuximab, temozolomide and/or intravenous BV. We included all imaging studies up to six months and then at 1 year post-treatment if available.
Brain MRI and DSC-MRI Data Collection and Processing
All neuroimaging examinations were conducted on a 3.0T HDxt 15x MR (GE Medical Systems, Mailwaukee, Wisconsin). Conventional MRI with a dedicated standardized SIACI BV imaging protocol (previously described) was performed.7 DSC-MRI data were acquired by using single-echo GRE echo planar imaging, with a flip angle of 60°; TR/TE of 2000/20 msec.; FOV: 240 mm; 129 × 96; ST/gap: 5 mm/0; NEX: 1; no. of shots: 1. The first 0.1 mmol/kg gadolinium administration was used as preload for the subsequent DSC study to correct the T1 weighted effects of vascular leakage on rCBV. Next, 0.10 mmol/kg gadolinium at 3–5 mL/s was administered at least 5 minutes after the preload injection.13, 14 The negative enhancement integration and linear fitting correction method was used for post-processing to calculate corrected rCBV and rCBF.13 Functional rCBV and rCBF maps were obtained and analyzed using Olea Sphere Version 2.3 SP2 (La Ciotat, France).
Selection of Regions of Interest and Evaluation of Data
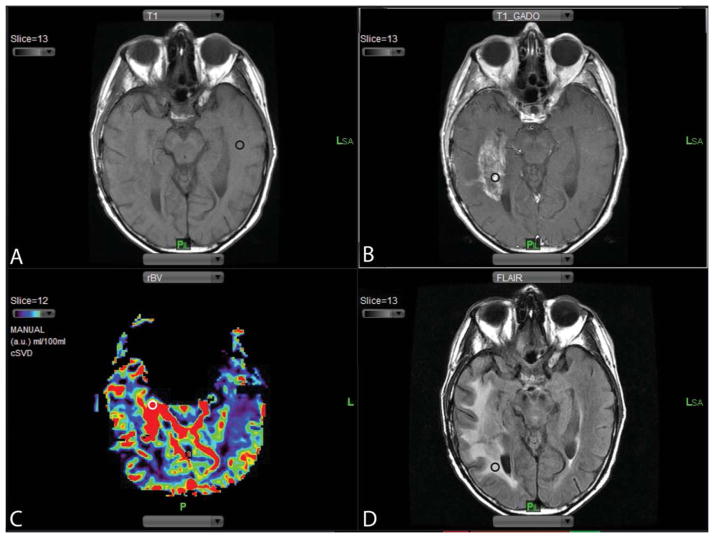
Up to four distinct regions of interest (ROIs) ranging in size from 10–12 voxels were chosen from the co-registered pre-contrast T1W, post-contrast T1W and T2-FLAIR images and rCBV maps (Fig 1): 1 – normal appearing white matter (NAWM) in the contralateral side (Fig 1A), which was used to normalize rCBV and rCBF maps on a voxelwise basis. (Normalized rCBV = rCBV (lesion)/ rCBV (NAWM); 2 – the location of highest T1 enhancement in the lesion (max enhancing) (Fig 1B), 3 – the location of highest rCBV in the lesion (max rCBV) (Fig 1C), 4 – non-enhancing T2 hyperintense signal abnormality surrounding the tumor (non-enhancing T2 hyperintensity) (Fig 1D). Same size and anatomically matching ROIs were manually constructed using contrast-enhanced T1W and T2W images as a reference from the pre- and post-treatment MRI scans. Only one investigator (S.K) placed ROIs and all ROI placements were overseen by two senior investigators (A.J.T and I.K.).
FIG 1.
ROI selection. (A) Pre-contrast T1W images are used to select the ROI in the normal appearing white matter (NAWM) in the contralateral side to the lesion (black circle). (B) Post-contrast T1W images are compared with the pre-contrast T1W images to select the ROI representing the area of max contrast enhancement (black circle). (C) Region of max rCBV is selected using rCBV maps (white circle). (D) T2-FLAIR images are used to select area representing the non-enhancing T2 hyperintense signal abnormality surrounding the tumor (black circle).
Statistical analysis
Differences in rCBV and rCBF from pre- to post-SIACI BV (defined as median percent change: [(post-treatment − pre-treatment) / pre treatment × 100%]) were determined using the Wilcoxon signed-rank test. Spearman’s correlation was used to assess the correlation between changes in rCBV and rCBF in the various ROIs and TTP and OS. Differences of rCBV and rCBF changes in ROIs were tested using ANOVA within subjects.
Results
DSC-MRP showed that SIACI BV produced changes in rCBV and rCBF (Fig 2, Table 2). Median percent change values are reported, which were not significantly different from the mean percent change values (Supplementary Table 1).
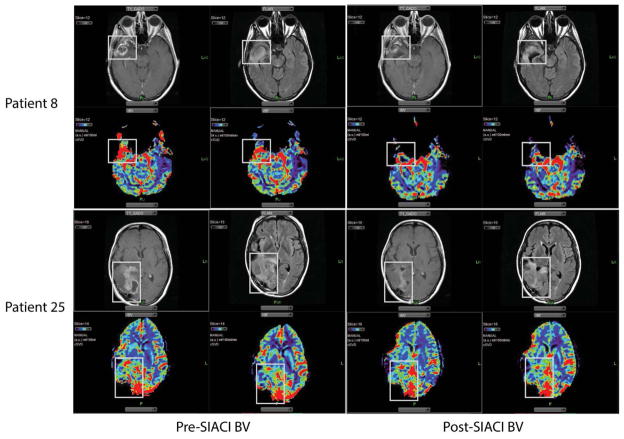
FIG 2.
MR changes from SIACI BV treatment. Imaging from two patients (study patients 8 and 25) demonstrates decrease in contrast enhancement, T2 signal abnormalities, rCBV and rCBF following SIACI BV.
Table 2.
Median Percent Change in rCBV and rCBF following SIACI
| rCBV* | rCBF* | |||||||
|---|---|---|---|---|---|---|---|---|
| Pt | Max Enhancing | Max rCBV | Non-enhancing T2 hyperintensity |
Contralateral NAWM |
Max Enhancing | Max rCBV | Non-enhancing T2 hyperintensity |
Contralateral NAWM |
| 1 | −66.30 | −74.39 | −50.59 | 20.55 | −56.93 | −63.82 | −37.19 | −46.12 |
| 2 | −34.35 | −69.71 | 158.48 | −20.00 | −43.04 | −67.05 | −20.67 | 38.11 |
| 3 | 66.63 | −14.11 | 80.50 | 22.73 | 31.44 | −25.47 | 46.15 | −11.79 |
| 4 | −52.79 | −13.79 | 27.75 | −32.56 | −45.03 | −15.25 | 20.99 | 67.22 |
| 5 | 16.890 | −58.09 | 136.91 | 14.75 | 12.55 | −56.74 | 145.63 | −11.15 |
| 6 | −27.49 | −7.41 | 16.62 | 2.94 | −30.04 | −16.82 | 42.84 | −17.12 |
| 7 | −43.07 | −79.18 | −42.58 | −57.14 | −31.80 | −76.40 | −28.00 | 51.61 |
| 8 | −39.46 | −39.46 | 76.22 | −4.94 | −47.56 | −47.56 | 113.73 | 29.26 |
| 9 | −31.86 | −47.62 | −61.32 | 143.75 | −31.56 | −45.45 | −44.37 | −23.63 |
| 10 | −20.00 | −33.93 | 5.95 | −22.22 | −12.29 | −30.67 | 11.16 | 5.24 |
| 11 | −33.45 | 2.38 | 37.55 | 10.38 | −28.05 | 13.66 | 35.38 | −20.99 |
| 12 | 117.64 | −53.85 | 96.31 | −2.00 | 55.60 | −66.57 | 63.62 | 75.74 |
| 13 | 21.69 | 38.90 | 184.63 | −82.35 | 15.65 | 40.36 | 158.46 | −56.60 |
| 14 | −34.28 | −40.98 | −13.83 | 196.55 | −37.52 | −45.97 | −14.74 | 50.83 |
| 15 | −28.08 | 34.83 | 30.96 | −4.26 | −30.70 | 44.18 | 15.46 | 0.36 |
| 16 | −57.80 | −36.72 | −33.07 | −6.76 | −54.27 | 3.25 | −21.19 | −2.98 |
| 17 | 51.42 | −22.99 | 157.76 | 4.29 | 34.75 | −23.78 | 149.41 | −10.09 |
| 18 | −1.16 | −29.24 | 255.42 | 50.63 | −21.11 | −40.68 | −65.88 | 68.95 |
| 19 | 1.12 | −4.39 | 23.08 | −13.33 | 21.18 | −9.21 | 23.35 | −68.77 |
| 20 | 7.54 | 21.19 | 30.04 | −17.20 | 6.00 | 25.52 | 21.70 | 1.59 |
| 21 | −4.01 | −32.24 | −6.81 | −13.33 | 0.99 | −26.78 | −3.42 | −0.91 |
| 22 | −32.84 | −12.87 | −83.26 | 0.000 | −27.25 | −9.19 | −73.03 | 50.63 |
| 23 | 40.87 | −38.70 | 217.37 | −6.59 | 47.83 | −45.15 | 208.97 | 3.99 |
| 24 | −25.30 | −64.33 | −61.21 | −26.25 | −19.91 | −61.04 | −63.85 | −1.19 |
| 25 | −31.29 | −7.75 | 145.08 | 28.57 | −66.00 | −49.42 | 61.25 | 33.46 |
| Median % Change (interquartile range) | −27.49 (−34.38 to 7.55) (−66.30 to 117.64) | −32.24 (−47.62 to −7.75) (−79.18 to 38.90) | 30.04 (−13.83 to 136.91) (−83.26 to 255.42) | −4.26 (−17.20 to 14.75) (−82.35 to 196.55) | −27.25 (−37.52 to 12.55) (−66.00 to 55.60) | −30.67 (−49.42 to −9.21) (−76.40 to 44.18) | 20.99 (−21.19 to 61.25) (−73.03 to 208.97) | 0.36 (−11.79 to 38.11) (−68.77 to 75.74) |
| P value for Median % Change+ | 0.074 | 0.001 | 0.069 | 0.568 | 0.037 | 0.001 | 0.216 | 0.696 |
NAWM – Normal appearing white matter; rCBF – relative cerebral blood flow; rCBV – relative cerebral blood volume; SIACI - superselective intra-arterial cerebral infusion
Listed values are calculated as median percent change = [(post treatment – pre treatment) / pre treatment × 100%])
Listed values are compared to 0% change (null value).
Cerebral Blood Volume
Comparing pre- and post-SIACI BV, there was a statistically significant median percent change of −32.34% (range: −79.18 to 38.90; p = 0.001) in rCBV in areas of max rCBV. There was a trend towards statistically significance in areas of max tumor enhancement (median percent change −27.29%; range: −66.30% to 117.64; p = 0.074) and in non-enhancing T2 hyperintense areas (median percent change 30.04; range: −83.26 to 255.42; p = 0.069). The change in rCBV was not found to be statistically significant in contralateral NAWM (median percent change −4.255; range: −82.35 to 143.75; p = 0.568). The median percent change in rCBV in non-enhancing T2 hyperintense region showed a trend towards statistically significant correlation with the presence of previous cycles of BV (p = 0.062). Median TTP and OS were 571 and 683 days, respectively. None of the rCBV changes correlated with prolonged TTP or OS. Lastly, the rCBV changes were significantly different between the four ROIs (p = 0.0003).
Cerebral Blood Flow
There was a statistically significant median percent change of −30.67 (range: −76.40 to 44.18; p = 0.001) and −27.25 (range: −65.99 to 55.60; p = 0.037) in rCBF in areas of max rCBV and max tumor enhancement, respectively, from pre- to post-SIACI BV. The change in rCBF was not found to be statistically significant in contralateral NAWM (median percent change 0.363; range: −68.77 to 68.95; p = 0.696) and in the non-enhancing T2 hyperintense areas (median percent change 20.99; range: −63.85 to 208.97; p = 0.216). None of the rCBF changes correlated with prolonged TTP or OS. Lastly, the rCBF changes were significantly different between the four ROIs (p = 0.021)
Discussion
Conventional MRI is currently unable to provide consistent and accurate assessment of pathology-specific tumor progression and therapeutic response, which limit its diagnostic and prognostic utlity.8 This limitation has led to the development of advanced quantitative imaging techniques that provide critical information on the molecular, physiological and metabolic processes and properties of tumors.15 Previously, we showed that MRSI, specifically Choline/ N-acetylaspartate ratios, provided a useful tool to assess treatment response following SIACI BV.7 In the current study, we used DSC-MRP to assess GB perfusion changes associated with SIACI BV in order to determine whether DSC-MRP provided useful biomarkers to determine treatment response. We also wanted to explore whether biomarkers obtained from DSC-MRI could reveal aspects of the complex mechanism underlying the tumoricidal effects of BV.
Anti-angiogenic agents such as BV produce a marked decrease in contrast enhancement, termed “pseudoresponse,” and a notable decrease in the non-enhancing T2 hyperintense areas. Standardized criteria for assessing brain tumor treatment response, including the Macdonald and the RANO criteria, fall short of definitively distinguishing tumor progression, pseudoresponse, and pseudoprogression.16 The inability of the Macdonald and RANO criteria to differentiate tumor progression, pseudoresponse and pseudoprogression is noteworthy and could lead to conflicting and confusing outcome evaluations in BV treatment. Recognition of pseudoresponse and pseudoprogression in anti-angiogenic therapy is critical to appropriately determine whether the decrease in contrast enhancement reflects a true decrease in tumor burden, or is simply due to normalization of BBB and tumor vasculature.
It remains unclear whether BV acts by pruning tumor vessels and killing a fraction of tumor cells, by normalizing existing tumor vasculature and the tumor micro-environment thus increasing the delivery of chemotherapy, or by reducing the number of blood-circulating endothelial and progenitor cells thus inhibiting neovascularization.17–19 MR diffusion weighted, perfusion weighted and spectroscopy may provide quantitative data on the molecular and metabolic processes that underlie tumorigenesis and tumor response. MRSI can be used to study neurochemical changes that may help explain the tumoricidal effects of BV.7 DSC-MRP offers another appealing parametric imaging technique to potentially elucidate the mechanism of action of BV.
DSC-MRP tracks the first pass of a bolus of gadolinium-based contrast agent through brain tissue by a series of rapid T2- or T2*-weighted MR images. The susceptibility effect of the paramagnetic contrast agent leads to a transient decreases in T2 and T2* relaxation times, resulting in signal loss in the signal intensity–time curve. The signal information can then be converted into a contrast medium concentration–time curve and used to generate parametric maps of rCBV, rCBF and K2 maps.20, 21 DSC-MRP is particularly sensitive to changes in tumor vasculature, which is noteworthy given that BV affects blood vessels. DSC-MRP therefore may be useful in both assessing tumor response to BV and in better understanding the tumoricidal effects of BV.
In the present study, DSC-MRP was used to assess tumor response in 25 patients with recurrent GB treated with SIACI BV. rCBV and rCBF were reliable biomarkers for assessing tumor response to SIACI BV. The change in rCBV from pre- to post-SIACI BV was statistically significant in the ROIs in max rCBV. The change in rCBV also showed a trend towards statistical significance in ROIs in max tumor enhancement, which was associated with an observable decrease in the lesion’s contrast enhancement. No statistically significant changes or trends were found in contralateral NAWM. The change in rCBF was statistically significant in ROIs in max rCBV and max tumor enhancement, and not statistically significant in ROIs in contralateral NAWM. Collectively, these data show that the SIACI BV acted locally at the site of tumor, with no significant effect in the contralateral NAWM. A recent study reported that perfusion decreased in ipsilateral and contralateral normal appearing brain after BV treatment.22 This study however obtained absolute CBV, and the route of BV administration was different from our study, which may explain the different findings.
In our patients, SIACI BV produced a marked decrease in rCBV and rCBF in the max rCBV and max tumor enhancing regions on DSC-MRP imaging. Interesting, we also observed a trend towards statistical significance in rCBV increase in the non-enhancing T2 hyperintense areas surrounding the lesion. This may suggest that while the contrast-enhancing region within the tumor may reflect the treatment response to SIACI BV, it may not adequately reflect tumor burden, treatment effect or tumor progression during or after SIACI BV treatment. It is unclear whether the increase in rCBV in non-enhancing T2 hyperintense region reflects an increase in tumor volume or perhaps an increase in tumor invasiveness. Since several pre-clinical and clinical studies have reported that anti-angiogenic therapy increases tumor invasiveness,23–25 the increased rCBV in non-enhancing T2 hyperintense region approximately one month post SIACI BV in our study may be reflective of this phenomenon. However, increased T2 hyperintensity occurs more commonly after long-term IV BV exposure and histologically represent a low-grade infiltrative phenotype. In our study, there was no statistically significant difference in TTP and OS among patients that received intravenous BV prior to SIACI BV to those that did not. Combined radiological and pathological correlative studies are needed to better understand the imaging biomarkers of tumor invasiveness, especially as they pertain to anti-angiogenic therapy.
Post SIACI BV changes in MRP biomarkers did not correlate with prolonged TTP and OS. It is difficult to conclusively state whether this was due to lack of treatment effect or due to other confounding variables. The sample size was small and clinical heterogeneity in patients selected for inclusion in the Phase I/II SIACI BV trials should be considered. Notably, over a quarter of our patients received exposure to BV prior to enrolling in SIACI BV clinical trials, and not every patient received the maximum dose of SIACI BV. Furthermore, over half of our patients received subsequent treatment post SIACI BV, making it difficult to accurately assess the true implications of this potential treatment. Given the design, the study carried limitations inherent to all retrospective reviews; namely, our results demonstrate correlation and not causation. The subjectivity in selecting matching ROIs on pre- post-treatment scans may have introduced sampling error. To minimize this, only one investigator (K.K) placed ROIs and all ROI placements were overseen by two senior investigators (A.J.T and I.K.). Another limitation was that histological specimens were not available to confirm the diagnosis of recurrent disease. While it is ideal to obtain histological specimens of recurrent disease, it is not realistic to expect patients to agree to an additional surgical procedure for open biopsy. Furthermore, even if a biopsy is obtained, correlation with post-treatment MRP changes may not be feasible since the exact site of biopsy is often not known or identifiable post biopsy, making it difficult to correlate MRP changes with histopathological examination. Future studies using SIACI BV should attempt to obtain biopsy specimens of recurrent disease using specified coordinates and match these coordinates voxel-by-voxel to post-SIACI BV treatment MRP scans.
Conclusions
This study suggests that GB response to SIACI BV can be assessed by comparing pre- and post-treatment rCBV and rCBF change in regions of the tumor with max rCBV and max enhancement. However, there was no correlation between these significant MRP biomarker changes, TTP and OS.
Supplementary Material
Acknowledgments
Funding: This work was partly supported by the Carolyn L. Kuckein Student Research Fellowship (R.S.), the RSNA Research Medical Student Grant (K.K) and the National Cancer Institute Grant No. CA130985 (J.A.B.)
ABBREVIATION
- BV
bevacizumab
- GB
glioblastoma
- IA
intra-arterial
- IV
intravenous
- OS
overall survival
- SIACI
superselective intra-arterial cerebral infusion
- TTP
time to progression
- VEGF-A
vascular endothelial growth factor-A
Footnotes
DISCLOSURES: Christopher Filippi—UNRELATED: Consultancy: Syntactx, Guerbet, Comments: Review of Brain MR Images for a Clinical Research Trial; Attended Advisory Board Meeting for Guerbet in Boston as consultant; Grants/Grants Pending: KIWH,* Coulter,* Comments: KIWH grant, co-investigator, funded 9/2015 to 8/2016 on grant determining optimum imaging strategy for women with acute stroke and Coulter Grant, PI, on development of novel semi-automated computer software alogrithm for core infarct detection.
money paid to institution
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Chinot OL. Bevacizumab-based therapy in relapsed glioblastoma: rationale and clinical experience to date. Expert review of anticancer therapy. 2012;12:1413–1427. doi: 10.1586/era.12.128. [DOI] [PubMed] [Google Scholar]
- 3.Mukherji SK. Bevacizumab (Avastin) AJNR American journal of neuroradiology. 2010;31:235–236. doi: 10.3174/ajnr.A1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riina HA, Fraser JF, Fralin S, et al. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. Journal of experimental therapeutics & oncology. 2009;8:145–150. [PubMed] [Google Scholar]
- 5.Boockvar JA, Tsiouris AJ, Hofstetter CP, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt JK, Riina H, Shin BJ, et al. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World neurosurgery. 2012;77:130–134. doi: 10.1016/j.wneu.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon JY, Kovanlikaya I, Boockvar JA, et al. Metabolic response of glioblastoma to superselective intra-arterial cerebral infusion of bevacizumab: a proton MR spectroscopic imaging study. AJNR American journal of neuroradiology. 2012;33:2095–2102. doi: 10.3174/ajnr.A3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay N, Waldman AD. Conventional MRI evaluation of gliomas. The British journal of radiology. 2011;84(Spec No 2):S107–111. doi: 10.1259/bjr/65711810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kickingereder P, Wiestler B, Burth S, et al. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro-oncology. 2015;17:1139–1147. doi: 10.1093/neuonc/nov028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmainda KM, Zhang Z, Prah M, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro-oncology. 2015 doi: 10.1093/neuonc/nou364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 12.Shin BJ, Burkhardt JK, Riina HA, et al. Superselective intra-arterial cerebral infusion of novel agents after blood-brain disruption for the treatment of recurrent glioblastoma multiforme: a technical case series. Neurosurgery clinics of North America. 2012;23:323–329. ix–x. doi: 10.1016/j.nec.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR American journal of neuroradiology. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology. 2008;249:601–613. doi: 10.1148/radiol.2492071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalpathy-Cramer J, Gerstner ER, Emblem KE, et al. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer research. 2014;74:4622–4637. doi: 10.1158/0008-5472.CAN-14-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RY, Neagu MR, Reardon DA, et al. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy - detecting illusive disease, defining response. Frontiers in neurology. 2015;6:33. doi: 10.3389/fneur.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falk AT, Barriere J, Francois E, et al. Bevacizumab: A dose review. Critical reviews in oncology/hematology. 2015 doi: 10.1016/j.critrevonc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature clinical practice Oncology. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 19.Okonogi N, Shirai K, Oike T, et al. Topics in Chemotherapy, Molecular-targeted Therapy, and Immunotherapy for Newly-diagnosed Glioblastoma Multiforme. Anticancer research. 2015;35:1229–1235. [PubMed] [Google Scholar]
- 20.Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: the five most frequently asked technical questions. AJR American journal of roentgenology. 2013;200:24–34. doi: 10.2214/AJR.12.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahng GH, Li KL, Ostergaard L, et al. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean journal of radiology. 2014;15:554–577. doi: 10.3348/kjr.2014.15.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer A, Pichler P, Karl M, et al. Quantification of serial changes in cerebral blood volume and metabolism in patients with recurrent glioblastoma undergoing antiangiogenic therapy. European journal of radiology. 2015 doi: 10.1016/j.ejrad.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 23.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncology. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.