Abstract
The carotid artery balloon injury model in rats has been well established for over two decades. It remains an important method to study the molecular and cellular mechanisms involved in vascular smooth muscle dedifferentiation, neointima formation and vascular remodeling. Male Sprague-Dawley rats are the most frequently employed animals for this model. Female rats are not preferred as female hormones are protective against vascular diseases and thus introduce a variation into this procedure. Left carotid is typically injured with the right carotid serving as a negative control. Left carotid injury is caused by the inflated balloon that denudes the endothelium and distends the vessel wall. Following injury, potential therapeutic strategies such as the use of pharmacological compounds and either gene or shRNA transfer can be evaluated. Typically for gene or shRNA transfer, the injured section of the vessel lumen is locally transduced for 30 minutes with viral particles encoding either a protein or shRNA for delivery and expression in the injured vessel wall. Neointimal thickening representing proliferative vascular smooth muscle cells usually peaks at 2 weeks after injury. Vessels are mostly harvested at this time point for cellular and molecular analysis of cell signaling pathways as well as gene and protein expression. Vessels can also be harvested at earlier time points to determine the onset of expression and/or activation of a specific protein or pathway, depending on the experimental aims intended. Vessels can be characterized and evaluated using histological staining, immunohistochemistry, protein/mRNA assays, and activity assays. The intact right carotid artery from the same animal is an ideal internal control. Injury-induced changes in molecular and cellular parameters can be evaluated by comparing the injured artery to the internal right control artery. Likewise, therapeutic modalities can be evaluated by comparing the injured and treated artery to the control injured only artery.
Keywords: Rat carotid artery, balloon injury, neointima, vascular disease, animal model, vascular smooth muscle cell hyperplasia, vascular wall remodeling
SHORT ABSTRACT
This protocol uses a balloon catheter to cause intraluminal injury on rat carotid artery and hence to elicit neointimal hyperplasia. This is a well-established model for studying the mechanisms of vascular remodeling in response to injury. It is also widely used to determine the validity of potential therapeutic approaches.
INTRODUCTION
Balloon catheters are medical devices used in the procedure of angioplasty, for the purpose of widening obstructed site(s) of atheroma or thrombus in a blood vessel The narrowed vessel lumen is forced to open up by the inflated balloon and blood supply would be restored sequentially to relieve downstream ischemia symptoms, such as angina, myocardial infarction, and leg pain. Nevertheless, the great success of angioplasty has been diminished by the post-operative complications as results from force-causes vascular barotrauma (balloon injury), namely vessel wall remodeling and in many cases re-narrowing of the vessel lumen (restenosis)1.
A number of animal models have been developed mimicking the angioplasty procedure to help investigators understand mechanisms underlying the balloon-injury-related vessel wall remodeling2. Among all the animal species utilized for modeling, rat is the most frequently used one. Compared to rabbits, dogs and swine, the advantages of rats are low cost, relative ease of use and current knowledge of rat physiology. Although mice have an added advantage in a wide range of genetically manipulated strains, the mice vessel is too small to insert a balloon catheter.. Over the past three decades, experimental rats have allowed researchers to gain better understanding of the molecular and cellular mechanisms underpinning neointima formation and vascular remodeling3–6. Beyond balloon injury, vascular remodeling are also involved in most major vascular diseases, such as atherosclerosis7, 8, hypertension9, and aneurysm10. Thus, knowledge gained through balloon injury model is in general beneficial to overall vascular wall disease studies.
The overall goal of rat balloon injury model is not only to further understand vascular diseases but also to test the potency of novel agents for disease control11, 12. Current clinical drug treatment to restenosis is applied by drug-eluting stents placed via the vessel lumen right after angioplasty. In animal models, an efficient yet more economical way for new agent testing is a well-developed local intraluminal perfusion method. Candidate agents that have been tested through this method include small molecule drugs13, 14, cytokine or growth factors15, 16, gene manipulating agents (cDNA clones, siRNA, etc)17–20, and novel pharmaceutical formulations21, 22.
So far, rat balloon injury model remains one of the most useful models for studying vascular diseases/disorders. It is the fundamental step from bench to bedside, usually as the first step moving from in vitro to in vivo, but it should not be the last one. Rat experiments’ outcome needs to be deliberated and further characterized before translation into human clinical use, due to the difference in vascular beds and vessel anatomy as well as the intrinsic species differences between human and rat.23–26. Nevertheless, it is still an essential tool in translational medical research. While such research used to be limited by lacking of genetically modified rats, it has been no longer an issue since novel genomic approaches such as zinc-finger nucleases27, TALENs28 and CRISPR-Cas29 have made easy access to knockout rat.
PROTOCOL
The use of animals for the following experiments has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
-
1
Preoperative procedures.
-
1.1
Sterilize surgical instruments before use.
-
1.1.1
Autoclave all surgical instruments 24 hours or less before surgery. If multiple surgeries are performed on the same day, sterilize the instruments by a dry bead sterilizer in between surgeries.
-
1.2
Filter-sterilize saline solution before use.
-
1.3
Weigh rat and calculate the dose of anesthetic drugs (ketamine 80 mg/kg and xylazine 7 mg/kg).
-
1.4
Administer the anesthetic drugs intra-peritoneally (i.p.).
-
1.4.1
Verify the adequacy of sedation by toe pinch in 5–10 min. Administer an additional small dose of drugs (ketamine 7 mg/kg and xylazine 0.6 mg/kg) if sedation is not complete.
-
1.4.2
Make sure the needle is deep enough to delivery drugs intra-peritoneally because failure to deliver the entire drug solution into the peritoneal cavity will cause inadequate sedation.
Note: Ulcers and hair loss in the skin at the injection site will be visible several days after surgery, due to inadvertent subcutaneous (s.c.) drug solution injection.
-
1.5
Inject 3 ml sterile saline solution subcutaneously (s.c.). Using a sterile cotton swab, apply a small amount of ophthalmic ointment to both eyes to prevent the corneas from drying out.
-
1.6
Prepare the heating equipment. Pre-warm heat pads by microwave or water bath.
-
1.7
Put the animal supinely on the surgical platform.
-
1.7.1
Remove the hair in the ventral neck region. Apply the hair remover with a cotton swab, wait 30 sec and wipe off completely with gauze.
-
1.7.2
Swab the neck with Povidone-Iodine scrub and 70% ethanol.
-
1.8
Put on personal protective equipment, including gown, hair cover, surgical mask, and glasses. Put on sterile surgical gloves at the end before handling the sterile surgical instruments and supplies.
-
1.9
Drape the rat with a sterile surgical sheet with only the neck region exposed.
-
2
Surgical procedures
-
2.1
During the surgical procedure, check sedation depth of animal by toe pinch in every 15 min. If animal responds to toe pinch, add additional small dose (10% of the initial dose) of ketamine and xylazine.
-
2.2
Dissect left common carotid artery (CCA)
-
2.2.1
Use a scalpel to make a straight longitudinal incision in the middle of neck.
-
2.2.2
Bluntly dissect the connective tissue from the skin. Keep the forceps tips up and make sure not to puncture the skin or the underlying tissue.
-
2.2.3
Dissect muscle layers longitudinally along the left side of trachea.
-
2.2.4
When opening up the muscle layer, visualize the left CCA with the vagus nerve closely attached. Bluntly dissect alongside the left carotid artery with extreme caution to separate the vagus nerve with minimal stretching.
-
2.2.5
Dissect CCA distally till the bifurcation. Carefully dissect the bifurcation and two branches - internal carotid artery (ICA) and external carotid artery (ECA).
-
2.2.6
Keep dissecting around the CCA, until approximately a portion of 1.5–2 cm of the artery is isolated from the surrounding tissues.
-
2.3
Balloon injury
-
2.3.1
Ligate on the ECA right at the proximal side of the superior thyroid branch. Ligate the occipital branch of ECA which is very close to the bifurcation of ECA and ICA. Clip on the proximal end of CCA and the distal end of ICA.
Note: Now, blood flow has been stopped either permanently through ligation or temporarily through clipping. Luminal contents in the bifurcation area have been isolated from the systemic circulation.
-
2.3.2
Make an arteriotomy incision on ECA by small-micro scissors. Ensure that the incision is close to the distal suture knot. Clean blood with saline and cotton swabs.
-
2.3.3
Insert the uninflated 2F balloon catheter into the ECA lumen. Advance the balloon catheter proximally to the CCA lumen. Keep advancing the catheter proximally until its tip reaches where the clip stays.
-
2.3.4
Connect the balloon inflation device to a female luer lock on a 3-way stopcock and connect the male luer lock of the 3-way stopcock to the balloon catheter.
-
2.3.5
Slowly inflate the balloon with approximately 1.5 atm pressure, in order to distend carotid artery to 1.5-fold of the diameter.
-
2.3.6
Gently pull the balloon rotationally back to the bifurcation.
-
2.3.7
Deflate the balloon and advance it back to the proximal end. Inflate it again and repeat the pulling-back procedure two more times.
-
2.3.8
Withdraw the balloon catheter from the artery lumen.
-
2.4
Intraluminal administration of reagents (e.g., siRNA, drugs)
In this case, the reagent we used was a solution containing lentiviral particles encoding either shRNA targeting Stromal Interaction Molecule 1 (STIM1) or non-targeting shRNA control. STIM1 is a single transmembrane endoplasmic reticulum (ER) protein that is an ER Ca2+ sensor controlling the activation of plasma membrane Ca2+ channels and is up-regulated during vascular smooth muscle dedifferentiation into a proliferative migratory phenotype12, 30–33.
-
2.4.1
Attach an intravascular over-the-needle catheter to a syringe. Aspirate 30 µl solution of the testing reagents. Insert the catheter into the same incision on ECA.
-
2.4.2
Advance the catheter tip into the CCA and tie a piece of suture with a single knot on the ECA to fix the catheter and close the incision temporarily.
-
2.4.3
Inject the testing reagent solution into the lumen of CCA. Keep the solution in the vessel lumen for 30 min. Keep the exposed tissue moist with saline and cover it with a piece of wet gauze.
-
2.4.4
After incubation, aspirate the remaining solution. Loosen that single knot and withdraw the catheter.
-
2.4.5
Tie the ECA with a piece of suture proximally to the arteriotomy incision. Make the knot as close as possible to the bifurcation.
-
2.5
Close up the wound.
-
2.5.1
Since the balloon injury and catheter introduction may cause a leak or puncture of the blood vessel, remove the clip on the ICA and check if there is any leak. If bleeding is observed, apply a piece of gauze and apply gentle pressure to stop bleeding.
-
2.5.2
Remove the other clip on CCA.
-
2.5.3
Make sure there are no signs of bleeding, and then remove all clamps and other surgical instruments. Cut off excess sutures.
-
2.5.4
Close wound using skin sutures. Swab on all sides of the closed wound with the Povidon-Iodine or other anti-septic/bactericide/virucide agent. Inject 3 ml of sterile saline s.c.
-
3
Postoperative Procedures
-
3.1.1
Keep rat on heat pad after surgery. Administer one dose of 0.05 mg/kg buprenorphine via intramuscular injection (i.m.) to the rat. During the recovery procedure, keep animals’ eyes and mouth moist. Monitor the animal until it is awake and ambulant.
-
3.1.2
Place the animal in a clean cage without any bedding until it is fully recovered. After recovery, the animal will be returned to the animal room and housed individually.
REPRESENTATIVE RESULTS
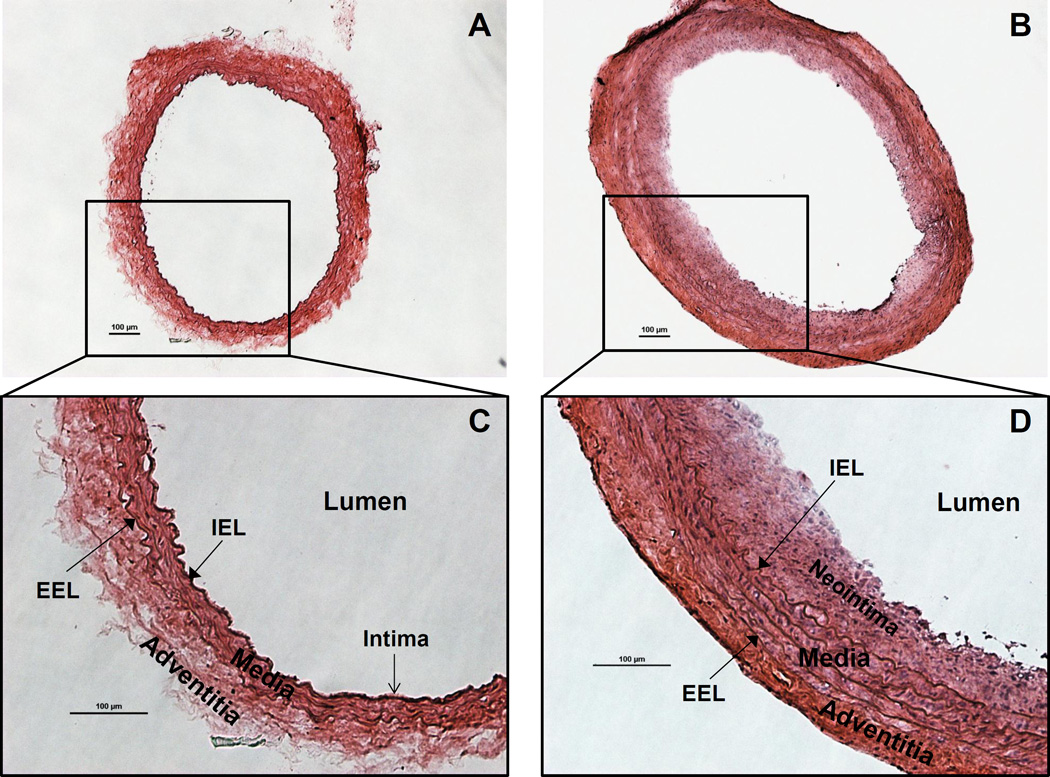
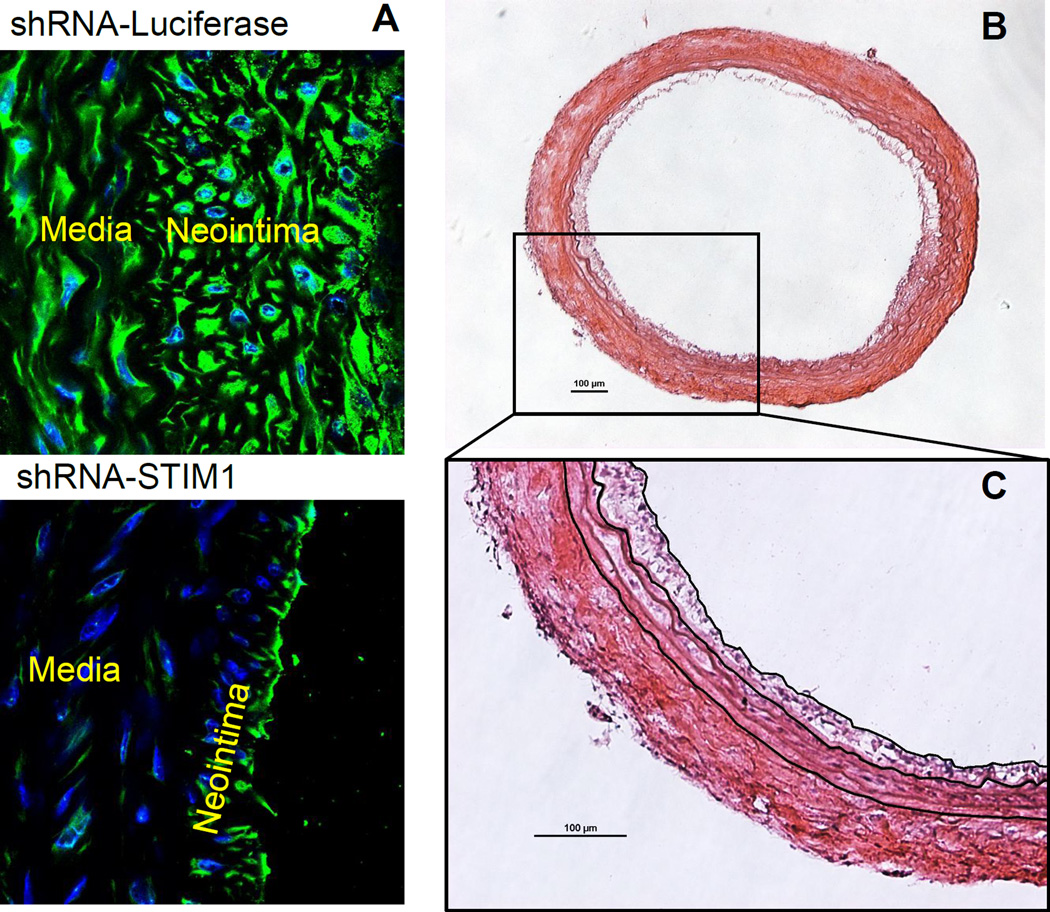
Two weeks after injury, carotid arteries are harvested, sectioned and subjected to morphological analysis. Arteries are cross-sectioned and stained with H&E (Figure 1, 2B, C and 3). Rat carotid artery wall contains four layers of elastic lamina, which appear as pink lines. Area between the outermost lamina, external elastic lamina (EEL) and the innermost lamina, internal elastic lamina (IEL) is the media smooth muscle layer (Figure 1). The area inside of the IEL is the intima, a monolayer of endothelial cells in intact vessels; or neointimal hyperplasia in injured vessel. In the injured carotid artery, the media is thicker than in control vessels due to proliferation of smooth muscle cells. The thickness of neointima is similar to or greater than the thickness of the media in the same artery. Adventitia is also thickened with robust collagen deposition (Figure 1B, D). For the artery treated with the reagent tested for its ability to inhibit neointima formation (in this case, STIM1 shRNA), the neointimal area of the cross section is smaller compared to the control injured artery (Figure 2). The validity of STIM1 knockdown with shRNA was evident as the STIM1 expression level largely decreased in the injured vessel neointima and media, compared to the control injured vessel.
Figure 1.
Rat carotid artery cross sections stained with Hematoxylin & Eosin (H&E). A. Morphology of the normal/intact (right) CCA. B. Morphology of the injured (left) CCA, showing neointima formation and adventitia/media thickening. C. Structure of the normal rat carotid artery wall. The intima is a monolayer of endothelial cells lining on the internal elastic lamina (IEL). Media is the smooth muscle cells and elastic tissues between IEL and external elastic lamina (EEL). Adventitia is the outer layer. D. Rat carotid artery wall robust thickening 2 weeks after injury. Smooth muscle cell proliferation and migration lead to neointima formation and media thickening. Typical concentric neointima formatted and media/adventitia thickened, showing successful generation of balloon injury phenotype. The red (eosin) staining is enhanced in adventitia, due to robust collagen synthesis. Scale bars are 100µm.
Figure 2.
Cross sections of rat carotid arteries with treatment of shRNA-STIM1 or shRNA control, two weeks post injury. A. Immunofluorescent staining of STIM1 and DAPI staining of nuclei on cross sections from both groups. The attenuated expression of STIM1 and neointima formation exhibit in the shRNA-STIM1 treated artery section. B. H&E staining of the shRNA-STIM1 artery section. C. Digital tracing of the neointima border, IEL and EEL, for the purpose of measuring areas of lumen, neointima and media. Scale bar are 100 µm.
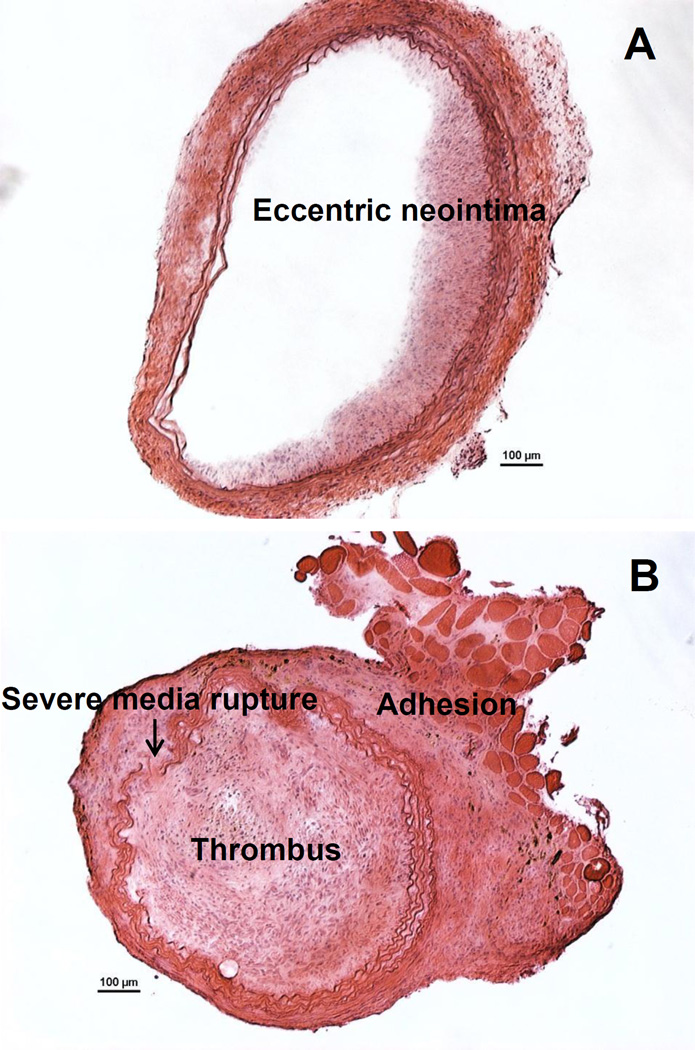
Figure 3.
Example of sub-optimal or failure to generate neointimal modeling. A. Eccentric instead of concentric neointima formed due to improper ballooning. B. Excessive injury (by an over-inflated balloon) caused severe damage to the vessel and the rupture of the vessel wall, which is evident by the discontinuous elastic laminas. Severe injury caused thrombus, which has blocked the entire vessel lumen and expanded out into the adventitia. Furthermore, enhanced adhesion occurred between the adventitia and the surrounding fat tissue. Scale bar are 100 µm.
As will be mentioned in Discussion section, surgeons should be cautious not to over-inflate the balloon and injure the vessel excessively. This would cause the vessel wall to rupture, which will cause blood leakage and robust thrombus formation both in the lumen and on the outer surface of artery, as shown in Figure 3.
DISCUSSION
The rat carotid balloon injury has been well described by Tulis in 200734. It has been comprehensively discussed all the details of this procedure by Dr. Tulis. The readers who are interested in performing this procedure are highly recommended to read Tulis’ protocol. However, there is one thing we are not agreeing with Dr. Tulis: Instead of inflating the balloon with saline or any kind of liquid, we suggested to inflate it with air. According to our personal experience, inflating with liquid can hardly avoid air bubbles. In addition, it is more difficult to flexibly adjust the pressure during surgery and may cause extra stress and injury to the artery. Another technique hint we would like to suggest here, is that using a small “pillow” made of paper towel or gauze is recommended to support animal’s neck during surgery.
Based on previous reports and several years of experience with rat balloon injury procedure, the authors have generated a slightly modified and simplified protocol35. In addition, the intraluminal infusion of therapeutics right after injury has been demonstrated. This has roughly doubled the time of this survival surgery procedure and thus requires additional hands-on experience. The major concern during the prolonged time period is the anesthetic plane. Initial dose of anesthetic drug can maintain rat’s sedation for 30–45 minutes and so the animal should be frequently checked by toe-pinch, especially during the 30 minutes-perfusion time. Also please note to avoid air bubbles in the vessel lumen during perfusion.
Like any other rodent surgeries, hypothermia is the major concern throughout the entire procedure. Use proper heating equipment to avoid animal suffering from hypothermia, which can cause death too. Meanwhile, over-heating/hyperthermia should also be avoided. When using the heat-pad, towels are recommended to be placed between the heat-pad and animal body to prevent the animal from getting too hot.
Two solutions, saline and lidocaine hydrochloride (1%) are highly recommended to be applied on exposed tissues when necessary during the surgical procedure. Tissues that exposed during surgery should be kept moist by sterilized saline. Surgical stretch frequently causes muscular spasm and vessel contraction of the carotid artery. Insertion of the balloon catheter into the contracted artery is prone to fail, or if succeed, will cause severe stretching or damage on the artery. Lidocaine as a topical anesthetic drug can be used to relax and dilate the vessel.
Balloon is inflated with approximately 1.5 atm pressure and proper adjustment is required for each surgery, due to the change in plasticity of aged balloon (if reused) and the variation of CCA diameter. In addition, due to stiffness of some arteries the balloon may be over-inflated but cannot be seen. In this case, when balloon is inflated, the surgeon should slightly pull the catheter to check the resistance of artery and adjust balloon pressure accordingly. Robust pulling will cause severe damage or rupture of artery, blood leakage, and failure of the experimental model. Balloon catheter can be reused several times as long as the balloon still works well. It is suggested to disinfect balloon catheter using Cidex for the purpose of reusing. The materials that made of balloon catheter, the natural rubber latex and polyethylene, have been approved to be compatible with Cidex (retrieved from http://www.hopkinsmedicine.org/hse/forms/cidexopa/OPAInstruction.pdf). The detailed protocols for disinfecting medical devices using Cidex have been described in the white paper of http://www.hopkinsmedicine.org/hse/forms/cidexopa/OPAHowToUse.pdf. It is important for the surgeon to check the leakage of balloon every time before use.
There are a variety of methods available for histological staining of tissue sections. H&E staining is the most commonly used one. Readers are referred to a comprehensively discussed article36, also written by Dr. Tulis, for further reading. In order to gain more accurate information of laminar structures, Verhoeff’s Elastic Tissue Stain with Van Gieson Counterstain (VVG staining) is highly recommended.
Acknowledgments
We thank Dr. Clowes for first developing and describing this method. Thank Dr. Tulis for his detailed protocol which has been fundamentally helpful to our previous, current and future work. This work was supported by grant R01HL097111 from the NIH, and by American Heart Association grant 14GRNT18880008 to M.T.
Footnotes
DISCLOSURES:
The authors declare that they have no competing financial interests.
Contributor Information
Zhang Wei, Nanobioscience, The State University of New York College of Nanoscale Science and Enginneering (SUNY CNSE), State University of New York, Albany, NY 12203, wzhang9@albany.edu.
Trebak Mohamed, Nanobioscience, The State University of New York College of Nanoscale Science and Enginneering (SUNY CNSE), State University of New York, Albany, NY 12203, mtrebak@albany.edu.
REFERENCES
- 1.Landzberg BR, Frishman WH, Lerrick K. Pathophysiology and pharmacological approaches for prevention of coronary artery restenosis following coronary artery balloon angioplasty and related procedures. Progress in cardiovascular diseases. 1997;39:361–398. doi: 10.1016/s0033-0620(97)80034-5. [DOI] [PubMed] [Google Scholar]
- 2.Muller DW, Ellis SG, Topol EJ. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992;19:418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- 3.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Laboratory investigation, a journal of technical methods and pathology. 1983;49:327–333. [PubMed] [Google Scholar]
- 4.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Laboratory investigation, a journal of technical methods and pathology. 1983;49:208–215. [PubMed] [Google Scholar]
- 5.Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury. IV. Heparin inhibits rat smooth muscle mitogenesis and migration. Circulation research. 1986;58:839–845. doi: 10.1161/01.res.58.6.839. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 7.Kiechl S, Willeit J. The natural course of atherosclerosis. Part II: vascular remodeling. Bruneck Study Group. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:1491–1498. doi: 10.1161/01.atv.19.6.1491. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto S, et al. Derivation of rat embryonic stem cells and generation of protease-activated receptor-2 knockout rats. Transgenic research. 2012;21:743–755. doi: 10.1007/s11248-011-9564-0. [DOI] [PubMed] [Google Scholar]
- 9.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 10.Meng H, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38:1924–1931. doi: 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun CK, Shao PL, Wang CJ, Yip HK. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: a review. American journal of translational research. 2011;3:259–268. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, et al. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circulation research. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ollinger R, et al. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 14.Levitzki A. PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovascular research. 2005;65:581–586. doi: 10.1016/j.cardiores.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91:2793–2801. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 16.Lee KM, et al. Alpha-lipoic acid inhibits fractalkine expression and prevents neointimal hyperplasia after balloon injury in rat carotid artery. Atherosclerosis. 2006;189:106–114. doi: 10.1016/j.atherosclerosis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Ji R, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circulation research. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 18.Merlet E, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovascular research. 2013;98:458–468. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Niu XL, Pippen AM, Annex BH, Kontos CD. Adenovirus-mediated intraarterial delivery of PTEN inhibits neointimal hyperplasia. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:354–358. doi: 10.1161/01.ATV.0000151619.54108.a5. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Cobos JC, et al. Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia. Circulation research. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman LA, et al. Local intraluminal infusion of biodegradable polymeric nanoparticles. A novel approach for prolonged drug delivery after balloon angioplasty. Circulation. 1996;94:1441–1448. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 22.Lipke EA, West JL. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta biomaterialia. 2005;1:597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Osterrieder W, et al. Role of angiotensin II in injury-induced neointima formation in rats. Hypertension. 1991;18:II60–II64. doi: 10.1161/01.hyp.18.4_suppl.ii60. [DOI] [PubMed] [Google Scholar]
- 24.Powell JS, et al. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989;245:186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- 25.Does the new angiotensin converting enzyme inhibitor cilazapril prevent restenosis after percutaneous transluminal coronary angioplasty? Results of the MERCATOR study: a multicenter, randomized, double-blind placebo-controlled trial. Multicenter European Research Trial with Cilazapril after Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MERCATOR) Study Group. Circulation. 1992;86:100–110. doi: 10.1161/01.cir.86.1.100. [DOI] [PubMed] [Google Scholar]
- 26.Faxon DP. Effect of high dose angiotensin-converting enzyme inhibition on restenosis: final results of the MARCATOR Study, a multicenter, double-blind, placebo-controlled trial of cilazapril. The Multicenter American Research Trial With Cilazapril After Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MARCATOR) Study Group. J Am Coll Cardiol. 1995;25:362–369. doi: 10.1016/0735-1097(94)00368-z. [DOI] [PubMed] [Google Scholar]
- 27.Geurts AM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesson L, et al. Knockout rats generated by embryo microinjection of TALENs. Nature biotechnology. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 29.Li D, et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nature biotechnology. 2013;31:681–683. doi: 10.1038/nbt.2661. [DOI] [PubMed] [Google Scholar]
- 30.Potier M, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aubart FC, et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. American journal of physiology. Cell physiology. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisaillon JM, et al. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. American journal of physiology. Cell physiology. 2010;298:C993–C1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulis DA. Rat carotid artery balloon injury model. Methods in molecular medicine. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Trebak M. T TRP Channels in Drug Discovery Methods in Pharmacology and Toxicology. 2012. pp. 101–111. [Google Scholar]
- 36.Tulis DA. Histological and morphometric analyses for rat carotid balloon injury model. Methods in molecular medicine. 2007;139:31–66. doi: 10.1007/978-1-59745-571-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]