Abstract
SHORT ABSTRACT
We describe how to deliver proteins and cell-impermeable small molecules into cultured mammalian cells by a simple co-incubation protocol with a reagent that causes endocytic organelles to become leaky.
LONG ABSTRACT
Macromolecular delivery strategies typically utilize the endocytic pathway as a route of cellular entry. However, endosomal entrapment severely limits the efficiency with which macromolecules penetrate the cytosolic space of cells. Recently, we have circumvented this problem by identifying the reagent dfTAT, a disulfide bond dimer of the peptide TAT labeled with the fluorophore tetramethylrhodamine. dfTAT, penetrates live cells by escaping from endosomes with a particularly high efficiency. By mediating endosomal leakage, dfTAT also delivers small molecules, peptides, and proteins into cultured cells after a simple co-incubation procedure. In this report, we describe the protocols involved in dfTAT synthesis and cellular delivery. We describe how to control the amount of protein that penetrates cells by varying the extracellular protein concentration while keeping dfTAT concentration constant. Finally, we discuss the current limitations of this new technology and steps involved in validating delivery. The described protocols should be extremely useful for cell-based assays as well as for the ex vivo manipulation and reprogramming of cells.
Keywords: Cell-penetrating peptide, cytosolic delivery, protein, fluorescence microscopy, cell culture
INTRODUCTION
The delivery of proteins, peptides or cell-impermeable small molecules into live cells is often desirable in many biological or biotechnological applications (cellular imaging, functional assays, cellular reprogramming, etc…)1–4. Many delivery approaches have already been reported, including microinjection, electroporation, or use of carrier agents (e.g. cell-penetrating peptides such as TAT, lipids)5–7. Each technique typically has specific pros and cons that might make these approaches adequate for certain applications but not for others. Common issues involve 1) poor delivery efficiencies and/or lack of control of how much material is delivered8, 9, 2) toxicity or deleterious physiological impact10, 11, 3) lack of temporal control, 4) delivery in few cells but not in a whole population (e.g. microinjection)12, 5) complex chemical conjugation or formulation schemes11.
Recently, we have developed a novel delivery strategy that circumvents these limitations. This strategy relies on a peptide named dfTAT (dimer fluorescent TAT)13. dfTAT is derived from the well-know cell–penetrating peptide (CPP) TAT. dfTAT contains two disulfide bonded copies of TAT labeled with the fluorophore tetramethylrhodamine. Despite their similarities, TAT and dfTAT differ significantly in activity. TAT is typically internalized into cells by endocytosis. This CPP remains however mostly trapped inside endosomes (this usually lead to a punctate distribution of the peptide inside cells when examined by fluorescence microscopy). Like TAT, dfTAT is efficiently endocytosed by cells. However, dfTAT does not stay trapped inside endosomes. Instead, it mediates endosomal leakage in a manner that is extremely efficient. The endosomolytic activity of dfTAT can then be exploited to deliver macromolecules by a simple incubation assay.
Our current understanding of the delivery process is as follows. dfTAT induces macropinocytosis. As a result, cells incubated with dfTAT take up soluble proteins, peptides, or small molecules (molecules of interest, MOI) present in media by fluid-phase endocytosis. Interactions between dfTAT and MOI are not necessary as long as both entities traffic together within the endocytic pathway. As dfTAT reaches a certain threshold within the lumen of endocytic organelles, it expresses its endosomal leakage activity (the molecular details remain to be fully characterized). The content of the lumen of leaky organelles, and therefore the MOI, is then released into the cells. This approach is therefore very convenient as no conjugation or formulation schemes with MOI are required. Moreover, because dfTAT is not directly modifying a MOI, it should also not interfere with MOIs function once intracellular delivery is achieved. In addition, the concentration of dfTAT used delivery is independent of that of MOI in media. For instance, dfTAT concentration can be kept constant between different experiments to guarantee reproducible efficiencies in endosomal leakage. In contrast, concentration of MOI in media can be gradually changed to achieve desired levels of MOI delivered in cytosol.
The high endosomal leakage efficiency achieved with dfTAT is remarkably innocuous to many of the cells tested to date. This is a surprise because endocytic organelles are important component of the cells and one would expect that the dramatic leakage mediated by dfTAT would be accompanied by deleterious cellular responses. Yet, treated cells proliferate at the same rate as untreated cells and do not display any significant changes in their transcriptome. Moreover, delivery can be repeated within minutes with reproducible delivery efficiencies, indicating that cells can either tolerate or recover from the delivery process without losing their capacity for endocytosis or endosomal leakage. Subtle cellular responses might take place during dfTAT delivery and the molecular details of what these responses might be remain to be explored. Yet, by combining high efficiency, convenience of protocols, and lack of toxicity, this delivery approach should prove immediately useful in many cell-based applications. The protocols presented herein are aimed at making this technology accessible to the research community.
PROTOCOL
1. SPPS: CK(TMR)TATG (fTAT) synthesis
dfTAT is produced in two steps: synthesis of the monomer fTAT by solid phase peptide synthesis followed by disulfide bond dimerization to form dfTAT
SPPS
-
1.1)
Rink amide MBHA resin (Novabiochem, San Diego, CA) is swelled in dimethylformamide (DMF) (Sigma Aldrich) in a standard SPPS vessel for 1 hour.
-
1.2)
Fmoc deprotection is performed by addition of piperidine in dimethylformamide (DMF) (Fisher, Waltham, MA) (20%, 10 mL) to the Fmoc-peptide resin (0.30 mmol). Deprotection reactions are carried out for 1 × 5 min and 1 × 15 min with a washing step in between reactions.
-
1.3)
CK(TMR)RKKRRQRRRG (fTAT) is synthesized on the rink amide MBHA resin by SPPS using standard Fmoc protocols. Fmoc-Lys(Mtt)-OH (only on N-terminus), Fmoc-Lys(Boc)-OH, Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Gln(Trt)-OH, and Fmoc-Cys(Trt)-OH (Novabiochem) are used to assemble the peptides. Reactions were carried out in a SPPS vessel at room temperature using a stream of dry N2 to provide agitation.
-
1.4)
Each amino acid coupling reaction is carried out for 4 h with a mixture of Fmoc-amino acid (1.2 mmol), HBTU (Novabiochem) (0.44 g, 1.1 mmol), and diisopropylethylamine (DIEA) (Sigma, St. Louis, MO) (0.51 mL, 3.0 mmol) in DMF. Upon completion of each reactions, the resin was washed with DMF and Fmoc deprotection was preformed as described in step 1.2. These steps were repeated until the linear peptide chain of fTAT was synthesized. The resin was then washed with DMF and followed by dichloromethane (DCM) (Fisher, Waltham, MA).
-
1.5)
Arginine is known to be an amino acid difficult to couple in SPPS. Successful coupling is etasblished by performing a Kaiser test14 (e.g. ninhydrin test). This test allows for the detection of free amines that might remain uncoupled on the resin. In case a blue color is detected (indicative of the presence of free amines), coupling steps are repeated.
-
1.6)
For CK(ε-NH-TMR)TATG (fTAT), the Mtt protecting group at the ε-amino group of Lys on CK(ε-NH-Mtt)TATG was cleaved with 2% trifluoroacetic acid (TFA) (Fisher) and 2% triisopropylsilane (TIS) (Sigma) in DCM, and the resin was washed with DCM and DMF.
-
1.7)
A mixture of TMR, HBTU, and DIEA (4, 3.9 and 10 equiv in respect to the peptide) in DMF was added to the resin and the reaction was carried out overnight using dry N2 to provide agitation.
-
1.8)
Following Fmoc-deprotection and peptide assembly, the resin was washed with DCM and dried in vacuo. The resin was then treated with TFA containing 2.5% H2O, 2.5%, TIS, and 2.5% ethanedithiol (EDT) (Sigma) for 3 h at room temperature to achieve global deprotection and cleavage from the resin. The crude peptide products were precipitated and washed with cold anhydrous Et2O (Fisher). The precipitates were resuspended in water and lyophilized. The products obtained were then resuspended in 0.1% aqueous TFA/acetonitrile.
-
1.9)
The peptides were analyzed and purified by reverse-phase HPLC. HPLC analysis was performed on a Hewlett- Packard 1200 series instrument and an analytical Vydac C18 column (5 μm, 4 × 150 mm). The flow rate was 1 mL/min, and detection was at 214 nm and 550 nm.
-
1.10)
Semi-preparative HPLC was performed on a Vydac C18 10 × 250 mm column. The flow rate was 4 mL/min, and detection was at 214 nm and 550 nm. All runs used linear gradients of 0.1% aqueous TFA (solvent A) and 90% acetonitrile, 9.9% water, and 0.1% TFA (solvent B).
-
1.11)
The correct identity of the peptides was confirmed by MALDI-TOF performed with a Shimadzu/Kratos instrument (AXIMA- CFR, Shimadzu, Kyoto). fTAT, expected mass: 2041.17, observed mass: 2040.66. DEAC-K9, expected mass: 1412.97, observed mass: 1415.59.
2. Oxidation Reaction: dfTAT generation
-
2.1)
Phosphate buffered saline pH 7.4 is aerated for 1 hour.
-
2.2)
fTAT (0.3 mg, 1.5×10−4 mmol) is dissolved in aerated phosphate buffer saline (PBS) pH 7.4 (5 mL). The oxygen dissolved in the buffer acts to oxidize the thiol groups on the peptides and form a disulfide bond.
-
2.3)
The reaction is allowed to react overnight until completion (100% yield based on HPLC analysis). The product is purified using reverse-phase HPLC and analyzed by mass spectrometry (MALDI-TOF). Expected mass: 4080.34, observed mass: 4084.21.
-
2.4)
Pure dfTAT is lyophilized and resuspended in MilliQ water.
3. Measuring dfTAT concentration
-
3.1)
An aliquot of the purified dfTAT (typically 1 μl depending on the amount of peptide purified) is resuspended in a 149 μl of 50 mM TCEP solution. In this step dfTAT is reduced to its monomer counterpart fTAT to eliminate the absorbance quenching that occurs due to the close proximity of the TMR fluorophore in dfTAT (the extinction coefficient ε of TMR in dfTAT is reduced in comparison to the ε of TMR in reduced fTAT).
-
3.2)
The sample is allowed to react for approximately 20 min (analytical HPLC can be used to confirm formation of fTAT).
-
3.3)
The solution is then added to the quartz cuvette and the absorbance is measured at 556 nm.
-
3.4)
Using Beer’s Law ( A= εcl : ε= 91,500 M−1 cm−1) to calculate the concentration of fTAT. To determine the concentration of dfTAT, divide [fTAT] by two.
4. Cellular delivery experiments
-
4.1)
The cells (HeLa, HDF, etc) are seeded in a dish (e.g. 8 well or 24 well dish).
-
4.2)
The cells are grown in appropriate medium (e.g. DMEM supplemented with 10% FBS and Pen/Strep) until 80–90% confluency in a 37ºC humidified atmosphere containing 5% CO2.
-
4.3)
The cells are washed three times (3X) with PBS.
-
4.4)
A 5 μM working concentration of dfTAT is made by diluting a stock of dfTAT (in MiliQ water) in nrL-15 media (for a 8 well dish the total volume should be 200 μL). A concentration of 5 μM dfTAT leads to efficient delivery (high level of cytosolic delivery in more than 90% of cells present in a dish) in most cell types tested to date (FIGURE WITH ALL CELLS TESTED). However, lower or higher concentrations might be more adequate for cell types with a high or low propensity for penetration.
NOTE ABOUT MEDIA: nrL-15 is used for the delivery of dfTAT since it does not contain cysteine which could reduce the disulfide bond in dfTAT. However, our data indicate that both regular L-15 (with cysteine) and DMEM can be used as the delivery media. L-15 contain the reducing amino acid cysteine but cysteine presumably oxidizes in the media to form cystine (DMEM is formulated with cystine). dfTAT therefore remains intact in these media and delivery works at the same efficiency as that obtained with nrL15.
-
4.5)
Cells are incubated with dfTAT with or without cargo (e.g. EGFP, TAT-Cre etc.) and kept at 37ºC for 1h (incubation time can be reduced but dfTAT typically requires approximately 30 to 45 min to induce endosomal leakage).
NOTE ABOUT CELLS:
Cells should not be overly confluent (> 90% confluency) since this might affect the delivery efficiency. Cells should also be healthy: dead cells in the culture can release apoptotic fragments with which dfTAT can interact (e.g. DNA from degraded nuclei). This in turn can interfere with delivery efficiency and the quality of imaging.
Cells should be washed thoroughly to remove FBS before adding dfTAT. BSA present in FBS can bind to dfTAT and this can lower the delivery efficiency of dfTAT. Washes should however be performed with care as excessive force might cause adherent cells to detach from the culture dish or cause a stress that results in lower endocytic uptake.
When delivering a protein/ peptide using dfTAT, the protein/peptide stock solution sample should be sufficiently concentrated to avoid excessive dilution of the nrL-15 media during incubation: e.g. adding on 5–10 μL of sample to 200 μL nrL-15 is recommended.
Delivery of some macromolecules with dfTAT could be problematic. For example, a protein containing an exposed region that is enriched in negative charges (or an MOI with a low PI) would result in dfTAT binding to the MOI (electrostatic interactions). This can in turn hinder its cell penetrating activity. Additionally highly positively charged species can compete with dfTAT and reduces it interaction with the plasma membrane of cells. This can therefore decrease the amount of dfTAT endocytosed by cells below the threshold required for efficient endosomal release.
-
4.6)
Cells are washed with heparin (1 mg/ml) in L-15 (3 washes are recommended) to remove dfTAT bound to the plasma membrane of cells.
-
4.7)
Cells are incubated with cell-impermeable nuclear stain such as Sytox Blue or Sytox Green (2 μM in nrL-15) to determine whether the plasma membrane of cells is compromised (dead cells will be stained while live cells will not).
-
4.8)
Cells are imaged using a fluorescence microscope (100X oil immersion or 20X objective). dfTAT is imaged using a RFP filter (Ex = 560 ± 20 nm/Em = 630 ± 35 nm). Successful delivery leads to a diffuse fluorescence of dfTAT throughout the cell (assessing the delivery of protein or peptide of interest will depend on application). Staining of nucleoli by fTAT (the reduced product of dfTAT upon cytosolic entry) can be used as an indication that the fluorescence detected is intracellular. fTAT will degrade within few hours. At this point, the fluorescence of the degradation fragments will appear as punctate. This should not be confused for the punctate distribution that is also seen if dfTAT remains unsuccessfully trapped inside endosomes (this can happen if dfTAT is present at too low of a concentration).
NOTE ABOUT IMAGING: fluorescently-labeled cell penetrating peptides can display a light-inducible membrane-disrupting activity.16 This is the case for dfTAT when high intensity light doses are used. It can manifest itself by rupture of intracellular organelles (e.g. endosomes, mitochondria), cell surface blebbing, and cell death. To minimize these effects, care should be taken to keep light exposure to a minimum (standard conditions required for imaging by confocal or epifluorescence are typically tolerated).
5. CONTROLING CONCENTRATION OF MOI DELIVERED
-
5.1)
Identify the “optimal delivery” concentration required to achieve efficient cytosolic release of dfTAT in the cell type used. This can be easily done by performing fluorescence microscopy on cells incubated with increasing concentrations of dfTAT. The optimal dfTAT concentration is defined as the minimal concentration that leads to clear cytosolic “diffuse” TMR fluorescence in approximately 100% of cells in a culture.
-
5.2)
Vary the concentration of MOI used in co-incubation protocol while keeping the concentration of dfTAT constant (e.g. using dfTAT’s optimal delivery concentration). Changing the incubation time to less than 1 hour could also be an option to vary the amount of MOI delivered.
DISCUSSION
When delivering MOI such as proteins, one is confronted to the problem that every macromolecule is unique and that, consequently, every delivery experiment might require fine-tuning (more so than in the case of DNA transfection where the molecules delivered are always a negatively charged polymer made of A, T, G, and C). Troubleshooting is therefore important and several aspects should be considered. First, as mentioned before, proteins with very low pIs can bind electrostatically to dfTAT and inactivate it. In contrast, proteins with very high pIs might compete with dfTAT for binding with negatively charged glycosaminoglycans on the cell surface. This might then reduce endocytosis and diminish uptake below endosomolytic thresholds. In both cases, a possible solution to this problem is increasing dfTAT concentration.
As one would expect, due to the presence of cellular proteases (such as cathepsins15) along the endocytic pathway, degradation of the MOI during delivery is a concern. While dfTAT can deliver intact proteins, the amount of protein that is fully or partially degraded during the delivery process has not been established. This is again an issue that is MOI-dependent and that should be monitored depending on the application pursued.
dfTAT is a highly efficient delivery agent. The molecular basis for dfTAT’s activity remains however unclear. In particular, the structural or chemical features that are required to achieve endosomal escape have not been identified. It is therefore currently not possible to predict how much the structure of dfTAT can be modified without altering delivery efficiencies. We have already established that the disulfide bond present in dfTAT can be replaced by a non-reducible linker without deleterious effects. Additional structure-activity relationships are currently being established.
Our data suggest that it is possible to decrease the incubation time of the peptide to less than one hour (as low as 5 min has been performed). However, the release of dfTAT inside cells is not observed in a large population of cells until approximately 15–30 min. This suggests that short incubation time may be sufficient for dfTAT endocytosis or cellular uptake. However time is required for endosomal maturation necessary for dfTAT escape from the endocytic pathway.
Finally, we have established multiple MOI can be delivered at once (using the protocols presented herein, with the only exception that more than one MOI is used during co-incubation). In addition, dfTAT-mediated delivery can be repeated (delivery steps repeated 20 min apart have been tested). This did not impact cell viability. We therefore believe that the delivery protocols presented herein are extremely convenient, efficient, and innocuous to cells.
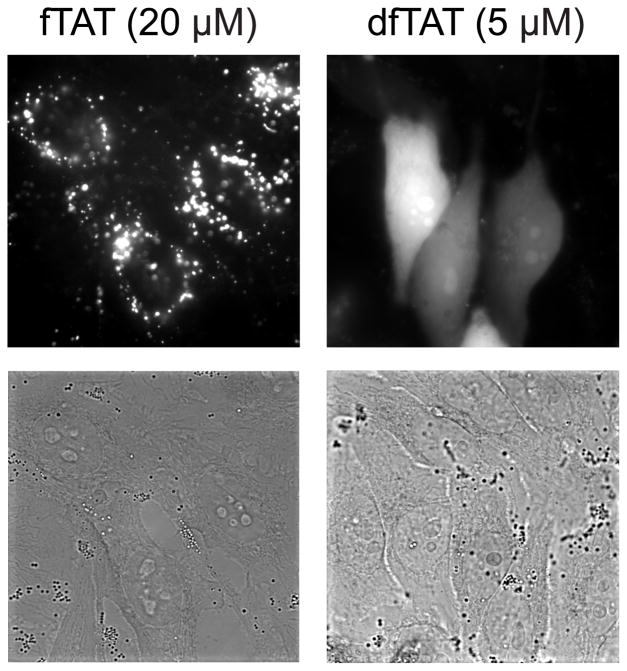
Figure 1.
Fluorescence (monochrome) and bright field images (100X objective) of HeLa cells incubated with 20 μM fTAT (monomeric fluorescently-labeled TAT, left panel) and 5 μM dfTAT (dimeric fluorescently-labeled TAT, right panel). The fTAT peptide displays a fluorescence punctate distribution while dfTAT exhibits a cytosolic and nuclear fluorescence distribution. Bright field images show no change in HeLa cell morphology upon peptide delivery. Scale bar, 10 μm.
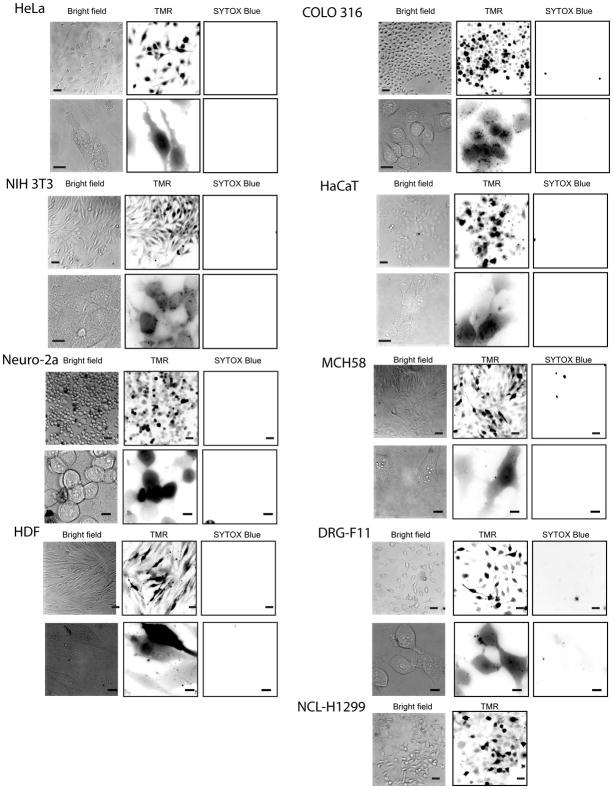
Figure 2.
Delivery of dfTAT into the cytosol and nucleus of live cells was achieved in multiple cell lines. The cell lines HeLa, NIH 3T3, COLO 316 and HaCaT, Neuro-2a, MCH58 and HDF were incubated with 5 μM dfTAT for 1 h, washed and imaged. The fluorescence signal detected was in the cytosol and nucleus of cells (top panel: 20X objective, bottom panel: 100X objective). After imaging, cells were incubated at 37 ºC in a humidified atmosphere containing 5% CO2 for 24 h, washed and imaged again (top panel: 20X objective, bottom panel: 100X objective). The cell morphology did not change after 24 h. Cell viability is assessed by exclusion of the cell-impermeable nuclear stain SYTOX Blue at both 1h and 24 h time point. The TMR fluorescence at the 24 h time point is different to that obtained at the 1 hctime point presumably because of the intracellular degradation of the peptide. Scale bars, 20X objective: 50 μm; 100X objective: 10 μm.
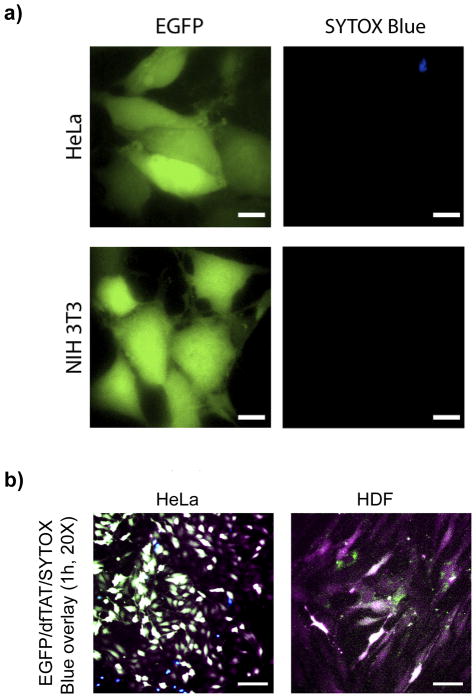
Figure 3.
dfTAT mediated delivery of intact EGFP into different cell lines. (a) HeLa (top panel) and NIH 3T3 (bottom panel) cells were incubated with EGFP (10 μM) and dfTAT (5 μM) for 1 h, washed and imaged. Images show a homogenous cytosolic fluorescence distribution of EGFP in HeLa and NIH 3T3 cells. Scale bars, 10 μm. (b) HeLa and primaryHDF cells were incubated with EGFP (10 μM) and dfTAT (5 μM) for 1 h, washed and imaged. Images show a homogenous cytosolic fluorescence distribution of EGFP and dfTAT in HeLa and primary HDF cells. Scale bars, 50 μm.
Acknowledgments
This article was supported by Award Number R01GM087227 and R01GM110137 from the National Institute of General Medical Sciences, the Norman Ackerman Advanced Research Program, and the Robert A. Welch foundation (Grant A-1769).
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/53175.
DISCLOSURES:
The authors have nothing to disclose
Contributor Information
Kristina Najjar, Email: kristina.najjar@tamu.edu, Biochemistry and Biophysics, Texas A&M University, College Station, U.S.A.
Alfredo Erazo-Oliveras, Email: alfredo_erazooliveras@tamu.edu, Biochemistry and Biophysics, Texas A&M University, College Station, U.S.A.
Jean-Philippe Pellois, Email: pellois@tamu.edu, Biochemistry and Biophysics, Texas A&M University, College Station, U.S.A.
References
- 1.Didenko VV, Ngo H, Baskin DS. Polyethyleneimine as a transmembrane carrier of fluorescently labeled proteins and antibodies. Analytical Biochemistry. 2005;344:168–173. doi: 10.1016/j.ab.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi T, et al. Direct and Rapid Cytosolic Delivery Using Cell-Penetrating Peptides Mediated by Pyrenebutyrate. ACS Chemical Biology. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- 3.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotech. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, et al. Reprogramming of somatic cells via TAT-mediated protein transduction of recombinant factors. Biomaterials. 2012;33:5047–5055. doi: 10.1016/j.biomaterials.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discovery Today: Technologies. 2008;5:e95–e103. doi: 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty P, Qian W, El-Sayed MA, Prausnitz MR. Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses. Nature nanotechnology. 2010;5:607–611. doi: 10.1038/nnano.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaczmarczyk SJ, Sitaraman K, Young HA, Hughes SH, Chatterjee DK. Protein delivery using engineered virus-like particles. Proc Natl Acad Sci U S A. 2011;108:16998–17003. doi: 10.1073/pnas.1101874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan C, Lu B, Chen H, Bishop C. Reprogramming human fibroblasts using HIV-1 TAT recombinant proteins OCT4, SOX2, KLF4 and c-MYC. Mol Biol Rep. 2010;37:2117–2124. doi: 10.1007/s11033-009-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fittipaldi A, et al. Cell Membrane Lipid Rafts Mediate Caveolar Endocytosis of HIV-1 Tat Fusion Proteins. Journal of Biological Chemistry. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Erazo-Oliveras A, Pellois JP. Delivery of macromolecules into live cells by simple co-incubation with a peptide. Chembiochem. 2010;11:325–330. doi: 10.1002/cbic.200900527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeles-Boza AM, Erazo-Oliveras A, Lee YJ, Pellois JP. Generation of endosomolytic reagents by branching of cell-penetrating peptides: tools for the delivery of bioactive compounds to live cells in cis or trans. Bioconjugate chemistry. 2010;21:2164–2167. doi: 10.1021/bc100130r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walev I, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci U S A. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erazo-Oliveras A, et al. Protein delivery into live cells by incubation with an endosomolytic agent. Nature methods. 2014;11:861–867. doi: 10.1038/nmeth.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 15.Lautwein A, et al. Human B lymphoblastoid cells contain distinct patterns of cathepsin activity in endocytic compartments and regulate MHC class II transport in a cathepsin S-independent manner. Journal of Leukocyte Biology. 2004;75:844–855. doi: 10.1189/jlb.0803367. [DOI] [PubMed] [Google Scholar]
- 16.Muthukrishnan N, Johnson GA, Lim J, Simanek EE, Pellois JP. TAT-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochimica et biophysica acta. 2012;1820:1734–1743. doi: 10.1016/j.bbagen.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]