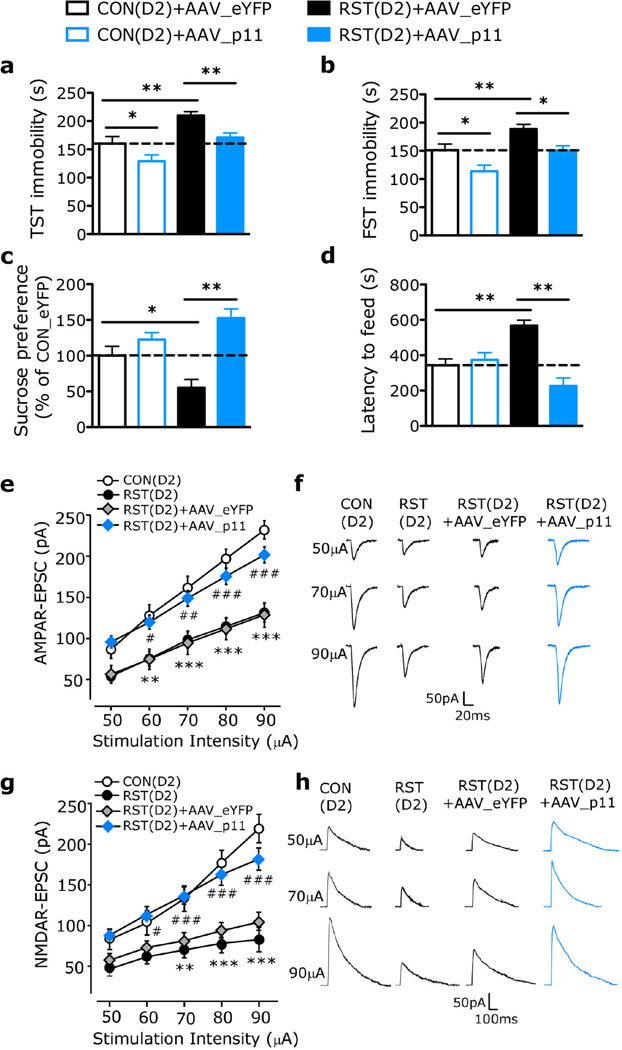
Figure 4. p11 overexpression in D2R-containing PrL neurons ameliorates stress-induced behavioral and glutamatergic synaptic deficits.
(a–d) Depression-like behaviors in control and stressed D2-Cre mice with the expression of AAV_p11 or AAV_eYFP in D2R-containing PrL neurons, as measured by TST (a), FST (b), SPT (c) and NSF (d) (n = 10 CON(D2)+AAV_eYFP, CON(D2)+AAV_p11; n = 12 RST(D2)+AAV_eYFP; n= 14 RST(D2)+AAV_p11). *P < 0.05, **P < 0.01, one-way ANOVA. (e,g) Summarized input-output curves of AMPAR-EPSC (e) and NMDA-EPSC (g) in D2+ layer II/III PrL neurons from control mice (D2-tdT) and RST mice with PrL injection of AAV_eYFP or AAV_p11 [e, n = 21 CON(D2), n = 26 RST(D2), n = 14 RST(D2)+AAV_eYFP, n = 22 RST(D2)+AAV_p11; g, n = 13 CON(D2), n = 13 RST(D2), n = 15 RST(D2)+AAV_eYFP, n = 12 RST(D2)+AAV_p11]. (f,h) Representative AMPAR-EPSC (f) and NMDAR-EPSC (h) traces in different groups. ** P < 0.01, *** P < 0.001, RST(D2) vs. CON(D2); # P < 0.05, ## P < 0.01, ### P < 0.001, RST(D2)+AAV_p11 vs. RST(D2)+AAV_eYFP, two-way ANOVA (e,g). CON(D2), control D2-Cre mice; RST(D2), D2-Cre mice exposed to chronic restraint stress. Data are means ± s.e.m.