Abstract
Currently, around 30% of patients with epilepsy do not have adequate seizure control. A greater understanding of the underlying mechanisms by which seizures start or propagate could lead to new therapeutic strategies. The recent development of optogenetics, due to its unprecedented precision for controlling activity within distinct neuronal populations, has revolutionized neuroscience, including epilepsy research. Here, we review recent breakthroughs made with optogenetics in epilepsy research. These include new insights into the key roles that different cell types play in mediating seizures as well as the development of epilepsy. Subsequently, we discuss how targeting different brain regions and cell populations has opened up the possibility of highly specific therapies, which can stop seizures on demand. Finally, we illustrate how combining newly available neuroscience tools with whole-brain imaging techniques will enable researchers to better understand the spread of seizures on a network level.
Introduction
Epileptic seizures are characterized by transient bursts of pathological activity permeating through brain networks. Since the middle of the 20th century, epilepsy researchers have used electrical and pharmacological tools to probe epileptic networks, leading to considerable improvements in treatments for epilepsy (Putnam and Merritt, 1937; Spiegel, 1937; Goddard et al., 1969; for excellent historical reviews of epilepsy drug development please see Shorvon, 2009a, 2009b; Brodie, 2010). However, around 30% of patients with epilepsy do not respond to anti-epileptic medications, and at this time we do not fully understand the conditions necessary for seizures to start, propagate or stop. The recent development of optogenetics, due to its unprecedented precision, has revolutionized neuroscience including epilepsy research: it has allowed us to target specific cell types and to switch them on or off with exquisite temporal control, revealing the details of seizure circuits like never before. While the use of optogenetics in epilepsy research is still in its infancy, exciting work has already emerged, including new insights into the key roles of certain cell types in mediating seizures and epileptogenesis. Optogenetics has also revealed the potential of very specific targeted interventions that could enable on-demand seizure interventions. Combining these new research tools with imaging technologies such as functional magnetic resonance imaging (fMRI) will enable us to have a better understanding of understanding seizure circuits over the whole brain. Here, we review some of the most recent breakthroughs in the field of optogenetics and epilepsy research. First, we discuss how optogenetics has been used to further our understanding of seizure circuits and following this, we consider the future potential of this technique to aid both our understanding and the treatment of epilepsy.
Understanding seizure circuits
There has been a long history in epilepsy research of using electrical stimulation of brain regions to drive seizure-like afterdischarges, and this technique is widely used in animal models (Goddard et al., 1969; Racine, 1972; Pinel and Rovner, 1978; Leung, 1987; Nissinen et al., 2000; Englot et al., 2008). However, electrical stimulation is non-specific, exciting all types of neurons as well as non-neuronal cells, thus making it difficult to elucidate the role played by individual cell types during the generation of seizures (Pitkänen et al., 2006). The optogenetics toolbox has helped to disentangle these roles and in this way has aided in our understanding of the conditions that enable seizures to emerge.
Optogenetics uses light at different wavelengths to excite or inhibit the activity of opsin-expressing cells with millisecond control (Boyden et al., 2005; Deisseroth, 2015). The non-selective cation channel channelrhodopsin-2 and its variants are used for depolarizing cells, and either halorhodopsin-3, a chloride pump, or Archaeorhodopsin-3, a proton pump, or their variants are commonly used for inhibition or hyperpolarization. By tying the expression of these genes to specific promoters, and delivering light onto targeted regions, the activity of particular cell populations can be rapidly modulated in a given circuit.
The emergence of seizures has classically been attributed to a shift in the balance between excitation and inhibition neuronal activity towards excitation. The mechanisms that underlie this transition to seizure are not well understood, but evidence suggests that there is significant interplay between pyramidal cells and interneurons in the emergence of epileptic phenomena. For example, in in vitro hippocampal slices from rats, simultaneous whole cell recordings from pyramidal cells and interneurons revealed that both types of cells are active at different times during the course of a seizure: interneurons are more active at onset of epileptiform discharges but then undergo a period of depolarization block, during which there is an increase in pyramidal cell firing (Ziburkus et al., 2006). Toyoda and colleagues found a similar response in vivo using unit recordings in the post-pilocarpine epileptic rat (Toyoda et al., 2015). Interestingly, the majority of interneurons recorded in the hippocampus (most notably in the subiculum) increased in firing rate up to four minutes prior to the onset of spontaneous seizures. During this time, a smaller population of interneurons decreased in firing rate. Following seizure onset, many of the examined interneurons briefly stopped firing before resuming activity. The mechanism of this pause is not known but Toyoda and colleagues speculated that it could be due to depolarization block resulting from elevated extracellular potassium ion concentrations.
The preictal change in interneuronal firing rate could indicate a possible role for them in the initiation of spontaneous seizures. In regards to evoked seizures, remarkably, blocking GABAergic synapses with bicuculline in hippocampal slices enhances the generation of short-duration interictal bursts but prevents electrically induced afterdischarges altogether (Higashima et al., 1996). When studying epileptic tissue from the human hippocampus, Huberfeld and colleagues found that the transition to seizure is preceded by pyramidal cell firing and dependent on glutamatergic signaling, whereas interictal epileptiform activity is preceded by interneuron firing and involves both glutamatergic and GABAergic mechanisms (Huberfeld et al., 2011). Nevertheless, because of complex interdependencies it has been difficult to disentangle the precise role of individual cell populations. The development of optogenetics has enabled researchers the possibility of driving specific cell populations with the aim of investigating their roles in seizure mechanisms.
Osawa and colleagues found that repetitive optogenetic stimulation of hippocampal neurons at 10 or 20 Hz efficiently evokes seizure-like afterdischarges in rats anesthetized under ketamine and xylazine (Osawa et al., 2013). Furthermore, selective stimulation of the excitatory hippocampal pyramidal cell population via the Ca2+/calmodulin-dependent protein kinase II α (CamKII) promoter (Weitz et al., 2015) also evokes seizure-like afterdischarges in awake and anesthetized rats, confirming the hypothesis that excessive tetanic stimulation of excitatory neurons can result in seizures. Alongside the benefits of inducing epileptic activity from specific cell types with temporal precision, another key advantage of optogenetic seizure initiation is that simultaneous electrophysiology remains uncontaminated by electrical stimulation artifacts during the stimulation period so that the dynamics of the stimulation period may be investigated. However, artefacts on electrophysiology can occur if the light delivered for stimulation hits the recording electrode, inducing a photovoltaic effect. These artefacts may be reduced or eliminated by moving the electrode away from light contamination (Cardin et al., 2010). Stimulating rat dorsal hippocampal neurons (expressing channelrhodopsin-2 under the Thy1.2 promoter) in vivo, Osawa and colleagues noted that at the start of stimulation, evoked potentials were time locked to the light pulses, but eventually abnormal spontaneous activity emerged in addition to the evoked potentials which persisted beyond the end of the stimulation period (Osawa et al., 2013). A Granger causality analysis on the LFP traces recorded from the septal and temporal hippocampus suggests that the direction of causal influence shifts during the development of afterdischarges from initially being greater in the septo-temporal direction at onset, to being greater in the temporal-septal direction towards the end. Using fMRI during selective stimulation of CamKII hippocampal neurons enabled the visualization of distinct frequency-dependent networks, which depended on the location along the septal-temporal axis at which the stimulation was applied (Weitz et al., 2015). For example, dorsal stimulations resulted in activity restricted to limbic regions whereas intermediate stimulations led to widespread brain activity, including recruitment of the cortical and subcortical regions. Awake-behavioral experiments also suggested a more severe phenotype upon stimulation of the intermediate compared to the dorsal hippocampus.
Modulating excitatory neurons is not the only means to drive seizures optogenetically and other research has suggested that activity within the inhibitory system also plays a critical role in this shift toward excitation. When postsynaptic intracellular chloride concentrations significantly increase, as occurs during seizure discharges, GABAA receptors become depolarizing instead of hyperpolarizing, and inhibitory interneurons effectively become excitatory, and this mechanism is thought to induce or facilitate seizures (Staley et al., 1995; Bernard et al., 2000; Lillis et al., 2012; Trevelyan et al., 2015). Consistent with this hypothesis, under certain conditions, epileptiform discharges can be initiated in entorhinal cortex slices by selective optogenetic activation of individual classes of interneurons, including parvalbumin (PV) and somatostatin (SOM) expressing neurons (Shiri et al., 2015; Yekhlef et al., 2015). For example, in the 4-aminopyridine (4AP) in vitro slice model, stimulating PV-expressing cells rapidly evokes epileptiform activity with a distinct low-voltage fast-onset pattern resembling those that occur spontaneously in the same model (Shiri et al., 2015). Similarly, Yekhlef and colleagues demonstrated that stimulating either PV- or SOM-expressing neurons in medial entorhinal cortical slices perfused in 4AP triggers interictal and preictal spikes which rapidly lead to seizure-like events (Yekhlef et al., 2015). Furthermore, these stimulations were accompanied by a rapid and transient accumulation of extracellular potassium, an effect also found in spontaneous events, before the emergence of epileptiform activity. Such an accumulation in itself can contribute to these discharges and is consistent with the hypothesis that intense interneuronal activation can result in an excitatory drive due to elevated intracellular chloride and a consequent increased extracellular potassium mediated by the K+-Cl− cotransporter KCC2 (Kaila et al., 1997; Viitanen et al., 2010; Yekhlef et al., 2015).
The role of inhibitory interneurons is likely to be nuanced and researchers have suggested that their effect is only apparent when there is a background of pathological activity (Ellender et al., 2014; Sessolo et al., 2015; Yekhlef et al., 2015). Activating PV interneurons in vitro only generated epileptiform discharges in areas with concomitant localized hyperactivity (Sessolo et al., 2015). Furthermore, directly increasing the GABA reversal potential by loading hippocampal pyramidal cells with chloride using halorhodopsin was not enough on its own to induce epileptiform discharges – although overall network activity was affected, discharges only emerged once a low dose of 4AP was added to increase background activity (Alfonsa et al., 2015). These findings suggest that the preictal environment is crucial in determining the role played by different cell types in seizure onset. Also highlighting the importance of the preictal state, in an in vivo model of absence seizures generated by optogenetic stimulation of excitatory neocortical neurons, Wagner and colleagues found the average LFP power before optogenetic stimulation appears to be significantly lower in trials that led to induced seizures compared to those that did not (Wagner et al., 2015). Likewise, the influence of different cell types may change, or wax and wane over the course of a seizure. For example, Ellender and colleagues found in their in vitro preparation that whereas PV interneurons are inhibitory during the early stages of a epileptiform discharges, during the later clonic stages they not only excite pyramidal cells because of high intracellular chloride levels, but also directly act to synchronize pyramidal cells across the network, thereby potentiating and maintaining epileptiform discharges (Ellender et al., 2014).
Epileptogenesis
Epileptogenesis is the process in which neural circuit reorganization leads to epilepsy. Understanding how the circuit reorganizes, and the conditions necessary for the emergence of seizures remain critical unsolved goals for epilepsy research (Buckmaster and Dudek, 1997; Kelley et al., 2009). One of the challenges to achieving these goals is that weeks, or even years, can pass between an epilepsy-inducing brain injury and the onset of seizures, and in between this time the insult has induced a myriad of processes, including inflammation, neurogenesis, cell death and gliosis (Mathern et al., 1995; Lukasiuk et al., 2003; Duffy et al., 2012; Choy et al., 2014a, 2014b). Interestingly, the degree of neuronal activity during development may play an important role by setting homeostatic limits that can influence subsequent seizure expression. In a genetic Drosophila model of epilepsy, researchers were able to prevent the emergence of the seizure phenotype by reducing neuronal activity with halorhodopsin during a critical period in embryonic development (Giachello and Baines, 2015). Conversely, by using channelrhodopsin to increase activity during this period, they were able to induce the seizure phenotype in wild type flies. If this property is conserved across species, identifying such a critical developmental window may have profound consequences for genetic epilepsies, and could also inform our understanding and treatment of acquired epilepsies, in which significant neurogenesis is known to occur.
Even after epilepsy emerges, however, it is unclear which features of an epileptic circuit make it hyperexcitable and inherently prone to seizures. Epilepsy is often associated with widespread circuit changes, but it is difficult to separate epileptogenic changes from those that are compensatory or mere epiphenomena. In temporal lobe epilepsy, common structural changes include mossy fiber sprouting, granule cell dispersion, loss of pyramidal cells in CA1 and changes to inhibitory neurons (Thom and Bertram, 2012) – but we do not yet know which of these changes, if any, makes the region prone to seizures. Directly manipulating individual cell populations will help to further characterize epileptic circuits and to reveal the roles of those cells (Buckmaster and Lew, 2011; Peng et al., 2013). For example, in rats with epilepsy induced by status epilepticus, there is substantial axonal reorganization in the hippocampus, including in dentate granule cells and in PV- and SOM-expressing interneurons (Peng et al., 2013). By targeting SOM-expressing CA1 neurons using optogenetic stimulation in slices taken from these epileptic rats, Peng and colleagues demonstrated that these interneurons increase their functional territories in the reorganized circuit, extending their influence from the CA1 into the dentate gyrus (Peng et al., 2013). Recently, three separate studies have shown that hippocampal grafts of GABAergic interneurons have been shown to reduce seizure frequency in chronic epilepsy (Hunt et al., 2013; Cunningham et al., 2014; Henderson et al., 2014). First, Hunt and colleagues showed that implanting inhibitory neurons into the mouse hippocampus dramatically reduced spontaneous seizures, while grafting these cells into the amygdala did not affect seizure frequency (Hunt et al., 2013). Cunningham and colleagues transplanted channelrhodopsin-2 expressing human derived maturing GABAergic interneurons into the hippocampi of epileptic mice and discovered extensive migration and integration with host circuitry (Cunningham et al., 2014). Channelrhodopsin-2 stimulation led to robust postsynaptic responses in host hippocampal neurons. Similarly, Henderson and colleagues transplanted channelrhodopsin-2-expressing fetal medial ganglionic eminence GABAergic progenitor cells into the mouse hippocampi 2 weeks following pilocarpine-induced status epilepticus, and found reduced seizure frequency during the period between 61 and 80 days following the initial injury (Henderson et al., 2014). These cells became functionally integrated into the network, and stimulating channelrhodopsin-2-expressing cells from hippocampal slices collected during this period yielded responses in host granule cells, innervated by these transplanted interneurons. Histology indicated that the grafts differentiated into interneuron subtypes including neuropeptide Y-, PV- and SOM-expressing cells. Further functional mapping of these grafts may reveal the connectivity changes that reduce seizure activity, and also help assess potential cell therapies for epilepsy.
Optogenetic seizure control
Controlling seizures, ideally without side effects, is still an unattained goal – around 30% of patients with epilepsy do not respond to conventional therapies. However, it has been shown that optogenetics can be used to ameliorate seizures, and that this can be achieved with only a brief targeted intervention at the beginning of the seizure (Krook-Magnuson et al., 2013; Paz et al., 2013).
Since the first study that reported halorhodopsin could be used to control hippocampal epileptiform activity in vitro in 2009 (Tønnesen et al., 2009), progress has been rapid and in vivo studies have quickly followed (Wykes et al., 2012; Krook-Magnuson et al., 2013; Paz et al., 2013; Sukhotinsky et al., 2013). Optogenetics has now been shown to be effective at ameliorating seizures in vivo across a spectrum of epilepsy models including neocortical, temporal lobe, post-stroke and absence epilepsies, as well as status epilepticus (see Table 1). For example, several studies have shown that halorhodopsin, expressed in excitatory neurons, can be used to stop seizures. Inhibiting neocortical CamKII-expressing neurons with halorhodopsin reduced epileptiform activity in the tetanus rat model of neocortical epilepsy (Wykes et al., 2012), and suppressing hippocampal activity was effective in a mouse model of temporal lobe epilepsy (Krook-Magnuson et al., 2013). Following photothrombotic cortical stroke in rats, using halorhodopsin to inhibit CamKII-expressing neurons in the ventrobasal thalamus halted thalamocortical seizures (Paz et al., 2013), and in the lithium-pilocarpine rat model of status epilepticus, inhibiting hippocampal CamKII-expressing neurons with halorhodopsin delayed seizure onset (Sukhotinsky et al., 2013).
Table 1.
A summary of regions targeted for optogenetic control of seizures in vivo
| Target region | Promoter | Cell type | Opsin | Model | Effect | Reference |
|---|---|---|---|---|---|---|
| Motor Cortex | CamKII | Glutamatergic | NpHR | Intracortical tetanus toxin | Reduced frequency of epileptiform events | Wykes et al. 2012 |
| Ventrobasal Thalamus | CamKII | Glutamatergic | NpHR | Photothrombotic stroke | Reduces power of epileptic events | Paz et al., 2013 |
| Hippocampus | CamKII | Glutamatergic | NpHR | Systemic pilocarpine-induced SE | Delayed SE onset by 5 min | Sukhotinsky et al., 2013 |
| Ipsilateral Hippocampus CA1 | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2013 |
| Contralateral Hippocampus CA1 | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2013 |
| Ipsilateral Hippocampus CA1 | CamKII | Glutamatergic | NpHR | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2013 |
| Ipsilateral Cerebellum | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2014 |
| Contralateral Cerebellum | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2014 |
| Ipsilateral Cerebellum | PV | GABAergic | NpHR | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2014 |
| Contralateral Cerebellum | PV | GABAergic | NpHR | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2014 |
| Midline Cerebellum (Vermis) | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration and increase time to next seizure | Krook-Magnuson et al. 2014 |
| Midline Cerebellum (Vermis) | PV | GABAergic | NpHR | Intrahippocampal KA spontaneous seizures | No effect on time to next seizure | Krook-Magnuson et al. 2014 |
| Hippocampus | PV | GABAergic | NpHR | Intrahippocampal KA spontaneous seizures | No effect | Krook-Magnuson et al. 2014 |
| Deep Cerebellar Nuclei | PV | GABAergic | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration and increase time to next seizure | Krook-Magnuson et al. 2014 |
| Purkinje Neurons in Ipsilateral Cerebellum | Pcp2 | Purkinje (GABAergic) | ChR2 | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2014 |
| Hippocampus | Thy1 | Pan-Neuronal | ChR2 | 4-AP intrahippocampal | Seizure activity (signal power) during stimulation reduced by 80% but does not stop events | Chiang et al. 2014 |
| Hippocampus | hSyn | Pan-Neuronal | NpHR | Intrahippocampal Bicuculline | Reduced bursting frequency | Berglind et al., 2014 |
| Ventrobasal Thalamus | CamKII | Glutamatergic | NpHR | Penicillin in somatosensory cortex | No effect | Han et al., 2015 |
| Ipsilateral Dentate Gyrus | Pomc | Granule cell | NpHR | Intrahippocampal KA spontaneous seizures | Reduces seizure duration | Krook-Magnuson et al. 2015 |
| Contralateral Dentate Gyrus | Pomc | Granule cell | NpHR | Intrahippocampal KA spontaneous seizures | No effect | Krook-Magnuson et al. 2015 |
| Ipsi/contralateral dentate gyrus | Pomc | Granule cell | ChR2 | Intrahippocampal KA spontaneous seizures | Increased seizure severity | Krook-Magnuson et al. 2015 |
| Superior Colliculus | hSyn | Pan-Neuronal | ChR2 | Systemic PTZ | Reduced seizure severity | Soper et al., 2015 |
| Superior Colliculus | hSyn | Pan-Neuronal | ChR2 | Bicuculline in area tempestus | Reduced seizure severity and frequency | Soper et al., 2015 |
| Superior Colliculus | hSyn | Pan-Neuronal | ChR2 | GEPR-3s (audiogenic seizure) | Reduced seizure severity and increased latency to onset | Soper et al., 2015 |
| Superior Colliculus | hSyn | Pan-Neuronal | ChR2 | Systemic Gamma butyrolactone (absence seizures) | Reduced seizure frequency and duration | Soper et al., 2015 |
| Hippocampus | Thy1 | Pan-Neuronal | ChR2 | 4-AP intrahippocampal | Seizure activity (signal power) during stimulation reduced but does not stop events | Ladas et al., 2015 |
| Cerebellar Nuclei | hSyn | Pan-Neuronal | ChR2 | Tg mice spontaneous SWDs | Reduced number of SWD events | Kros et al., 2015 |
| Cerebellar Nuclei | hSyn | Pan-Neuronal | ChR2 | C3H/HeOuJ mice spontaneous SWDs | Reduced number of SWD events | Kros et al., 2015 |
| Dentate Gyrus | Vgat | Interneurons | ChR2 | KA intrahippocampal SE | Reduced seizure frequency post-KA and stopped seizure propagation to MEC and motor cortex | Lu et al., 2016 |
| Medial Entorhinal Cortex (MEC) | Vgat | Interneurons | ChR2 | KA intrahippocampal SE | Stopped seizure activity in MEC but did not stop seizure activity in dentate gyrus and M1 | Lu et al., 2016 |
| Dentate Gyrus | Gad | Interneurons | ChR2 | KA intrahippocampal SE | Reduced seizure frequency post-KA and stopped seizure propagation to MEC and motor cortex | Lu et al., 2016 |
| Dentate Gyrus | Gad | Interneurons | NpHR | KA intrahippocampal SE | No effect on seizures | Lu et al., 2016 |
Abbreviations: 4-AP - 4-aminopyridine, CamKII -Ca2+/calmodulin-dependent protein kinase II α, ChR2 – channelrhodopsin2, Gad- glutamate decarboxylase, hSYN – human synapsin, KA – kainic acid, NpHR – halorhodopsin, Pcp2- purkinje cell protein 2, Pomc – proopiomelanocortin, PTZ – pentylenetetrazol, PV – parvalbumin, SE – status epilepticus, Thy1 – thymocyte differentiation antigen 1, Vgat – vesicular GABA transporter.
Another approach for suppressing seizures in vivo is to selectively activate interneurons with channelrhodopsin-2 (Krook-Magnuson et al., 2013; Lu et al., 2016). For example, Krook-Magnuson and colleagues reported that selectively stimulating PV interneurons reduced seizure duration by 43% compared to within animal non-stimulated control (Krook-Magnuson et al., 2013). However, stimulating larger populations of interneurons in vivo may further improve efficacy as suggested by Ledri and colleagues: stimulating Gad2-ChR2 neurons, which include PV, SOM, neuropeptide Y, and cholecystokinin interneuronal subpopulations, was more effective at suppressing epileptiform activity in slice preparations than either PV or SOM interneurons alone (Ledri et al., 2014). Although a direct comparison between targeting mixed and specific interneuron populations for in vivo seizure control has yet to be performed, stimulating mixed interneuron cell types in vivo has been shown to be effective at controlling intrahippocampal KA-induced acute seizures (Lu et al., 2016). Also, there exists the possibility that both halorhodopsin and channelrhodopsin may be less effective at the later stages of seizures, due to the accumulation of intracellular chloride (Huberfeld et al., 2007; Ellender et al., 2014; Alfonsa et al., 2015; Soper et al., 2015). One potential means of suppressing activity without directly modulating intracellular chloride is the opsin archaeorhodopsin, an outward proton pump (Raimondo et al., 2012; Pavlov et al., 2013; Alfonsa et al., 2015). However, although archaeorhodopsin has been effective at stopping epileptiform activity in vitro, there remain no published reports of its effects on seizures in vivo at the time of writing.
For each form of epilepsy, multiple regions may be good targets for seizure control, and it is important to understand which are the most effective, with the fewest side effects. For example, in a mouse model of temporal lobe epilepsy, Krook-Magnuson and colleagues have reported that spontaneous seizures could be controlled in regions including the ipsilateral and contralateral hippocampi, ipsilateral dentate gyrus, and in the cerebellum. Notably, however, targeting PV neurons in the vermis of the cerebellum not only successfully stopped seizures on-demand, but also increased the time between seizures (Krook-Magnuson et al., 2014). Furthermore, targeting the cerebellum may also be effective at controlling absence seizures (Kros et al., 2015). Whether the cerebellum, or other regions such as the superior colliculus (Soper et al., 2015), can generally alleviate seizure activity clinically remains to be seen, but identifying regions with general anti-seizure properties would be particularly useful for treating refractory epilepsies, such as multi-focal epilepsies, in which surgery is not an option (Chen et al., 2015; West et al., 2015). Optogenetic seizure control has exciting potential for epilepsy therapy, but it is important to note that it is still very early days for optogenetics, and there will be significant hurdles before translation to the clinic becomes a reality. In contrast, deep brain stimulation (DBS) has a longer clinical history, and has been recently approved for seizure control in the clinic (Morrell, 2011; Fisher and Velasco, 2014; Bergey et al., 2015). Although the mechanisms that underlie DBS are not well-understood, studies suggest that the regions chosen for stimulation and the choice of stimulation parameters determine its efficacy (Lozano and Lipsman, 2013). Thus, in the short-term, one promising application of optogenetics research may be to inform the location and stimulation parameters that will be most effective for seizure control using DBS (Chiang et al.; Fisher and Velasco, 2014; Bergey et al., 2015; Ladas et al., 2015).
Imaging seizure networks
Optogenetic control of seizures holds much promise as a potential therapy, but little is known about the consequences that these focal interventions may have on seizure networks and underlying brain function. Evaluating seizure activity and its effects across the whole brain in vivo will therefore help optimize treatments and limit potential off-target effects (Faingold and Blumenfeld, 2015). Combining optogenetics with imaging tools like functional magnetic imaging (fMRI) (Lee et al., 2010; Desai et al., 2011; Kahn et al., 2011; Duffy et al., 2015; Liu et al., 2015; Weitz et al., 2015) or positron emission tomography (Kolodziej et al., 2014) has significant potential as a means of understanding these seizure networks in greater detail.
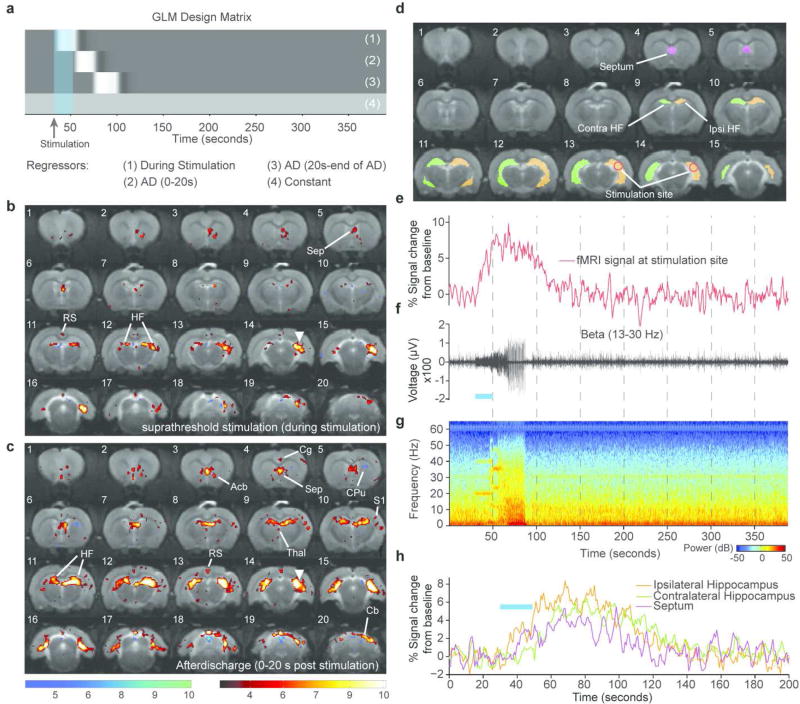
Visualizing activity over the whole brain can be achieved by using fMRI as a readout and optogenetic stimulation to modulate activity (Lee et al., 2010; Duffy et al., 2015; Weitz et al., 2015). For example, stimulating the intermediate and dorsal hippocampus has been shown to evoke activity in distinct networks (Weitz et al., 2015). Whereas intermediate hippocampal stimulation resulted in widespread activity that included cortical and subcortical regions, stimulating the dorsal hippocampus resulted in activity restricted to the limbic system. Notably, dorsal hippocampal stimulation resulted in decreased fMRI signal in the contralateral dentate gyrus. This response may indicate the presence of seizure modulating activity attributed to the dentate gyrus. Because seizures and often detected and classified using physiology, simultaneous measurement of electrophysiological signatures allow confirmation of seizures using well accepted measures (Logothetis, 2002; Kim and Ogawa, 2012). By developing MRI compatible optrodes for simultaneous LFP and fMRI in rats sedated using dexmedetomidine, we were able to show that stimulating hippocampal CamKII-expressing neurons with channelrhodopsin-2 (using a sub-seizure threshold light intensity) results in activity restricted to the ipsilateral hippocampus and septum. However, when the light intensity delivered to the brain was increased, seizure-like afterdischarges were generated and were confirmed using fMRI and electrophysiology together. The fMRI time series results in a prolonged hemodynamic response during afterdischarges and simultaneously acquired LFP measurements enables a more informed analysis of the fMRI data. During afterdischarges, the activated network extended to the contralateral hemisphere, recruiting the hippocampus, septum and cerebellum, as well as discrete cortical and thalamic regions (Duffy et al., 2015)(see Fig. 1). These networks are similar but distinct from those reported following electrical hippocampal stimulation (Englot et al., 2009).
Figure 1. Single subject simultaneous LFP and optogenetic fMRI during seizure-inducing (suprathreshold) stimulation of the hippocampus.
(a) GLM design matrix for the fMRI analysis. (b) T-statistic map showing regions of significant BOLD signal change during a seizure-inducing stimulation (average of 2 trials). (c) T-statistic map showing regions of significant BOLD signal change during the first 20 s an epileptiform afterdischarge. Site of optical stimulation is marked by the white triangle. (d) Segmentation of 4 different ROIs. (e) fMRI time course shown for a single trial. (f) Single trial simultaneously recorded LFP shown for the Beta band 13–30 Hz. (g) Spectrogram of the LFP recording during fMRI acquisition. (h) fMRI time course for the single trial shown from the ipsilateral hippocampus, septum and contralateral hippocampus. Duration of optical stimulations are marked by blue bars. T-statistic maps are thresholded at a significance level of p<0.01, voxel-wise FDR corrected. Abbreviations: Acb - Accumbens Nucleus, Cpu - Caudate Putamen, RS - Retrosplenial Cortex, Thal - Thalamus, Cg - Cingulate Cortex, HF - Hippocampal Formation, S1 - Primary Somatosensory Cortex, Sep - Septum. (Reproduced from Duffy et al., 2015, Copyright (2015) with permission from Elsevier)
During electrically-induced afterdischarges, fMRI signal changes are observed in the hippocampus and septal nuclei, while reduced neuronal activity results in the cingulate, retrosplenial and orbital frontal cortices. Genetically targeting only neuronal cells has the advantage of being able to evoke a hemodynamic response that is more physiological and less affected by stimulation of non-neuronal cells. Notably, despite seizures being evoked under a non-epileptogenic background the activated regions encompass those that have been shown to be effective targets for controlling temporal lobe seizures (Krook-Magnuson et al., 2013, 2014, 2015), demonstrating the potential that imaging seizure networks may have for identifying regions for seizure control. Nevertheless, the aforementioned studies imaged evoked seizures in a normal non-epileptogenic network. Since epilepsy is defined by significant alterations in the underlying networks, imaging seizures in a pathological setting may identify additional changes that may not be apparent in seizures evoked in a naïve brain.
While many regions may be activated during a seizure, it is unlikely that all of these regions may be equally efficacious for seizure control (see Table 1). As a seizure starts and spreads through a network, targeting regions early in the propagation pathway may be more effective at abolishing the seizure than regions recruited later. Using a focal hippocampally-evoked seizure mouse model and multi-regional electrophysiological recordings, Lu and colleagues found that seizures propagated from dentate gyrus to medial entorhinal cortex (MEC) (Lu et al., 2016). When interneurons (by targeting Vgat or Gad2) in the dentate gyrus were stimulated, seizure activity in dentate gyrus, MEC and motor cortex stopped. However, when interneurons in the MEC were stimulated, seizure activity was abolished in that region but persisted in the dentate gyrus and the motor cortex, demonstrating that successfully controlling seizure activity within a region does not necessarily indicate effective seizure control. Therefore imaging seizure networks and their interactions with proposed seizure control interventions can help identify critical nodes that are effective for completely stopping seizures.
Imaging seizure networks has also enabled researchers to identify a signature pattern in fMRI and electrophysiological recordings which is thought to be associated with the impaired consciousness that occurs during seizures (Blumenfeld, 2012; Motelow et al., 2015) or during stimulations that regulate arousal (Liu et al., 2015). Impaired consciousness during seizures is known to be associated with prominent frontoparietal slow wave activity on electrophysiological recordings in humans (Englot et al., 2010). Using fMRI in rats has enabled researchers to investigate the underlying circuit mechanisms of this phenomenon (Englot et al., 2009; Motelow et al., 2015;). These data indicate a possible role for the lateral septum and anterior hypothalamus which are activated during fMRI acquisitions and are known to have inhibitory connections with subcortical arousal systems. Using mice anesthetized with ketamine and xylazine, Furman and colleagues reduced this cortical slow wave activity during electrically evoked hippocampal seizures by optogenetically stimulating cholinergic brainstem neurons, suggesting that targeting these circuits may be effective for restoring consciousness (Furman et al., 2015). Thus imaging seizure networks are important for allowing epilepsy researchers to go beyond stopping seizures, to identifying regions that may not directly form part of the seizure network but have pathological implications for brain function.
Summary
Optogenetics has proven to be a useful set of tools for epilepsy research with powerful potential applications in the clinic. However, many questions still remain. For example, there is still little that is known about many aspects of epilepsy circuits, including the role of sub-classes of interneurons, glia, and G-protein coupled receptors such as metabotropic glutamate or GABAB receptors (Craig and McBain, 2014; Kubota, 2014; Roux et al., 2014). Excitingly, the breadth of optogenetic tools continues to expand rapidly and now includes opsins that can be excited with different wavelengths, that have improved kinetics or that can be switched on for extended periods with a single light pulse and switched off with another (Mattis et al., 2012). Furthermore, engineered opsins now come as inhibitory chloride channels or G-protein coupled receptors, and can even directly modulate gene expression and epigenetic processes (Konermann et al., 2013; Levitz et al., 2013; Berndt et al., 2014). Targeting specific cell populations using optogenetics can help us to understand the generation and propagation of seizures as well as the development of epilepsy. Modulation of circuits to interrupt seizure activity has exposed the possibility of highly specific anti-epileptic strategies. Finally, combining these new techniques with whole brain imaging methods can be used to observe seizure dynamics over the whole brain, and during modulation of distinct neural circuits. Such imaging approaches can be used to identify specific nodes in networks, as well as time points which might be crucial for seizure propagation or even the development of epilepsy. Thus optogenetics, in combination with the many other new technologies being developed, will likely pave the way for significant strides in our understanding and treatment of epilepsy in the near future.
Significance Statement.
Optogenetics is a relatively new technique which enables researchers to control brain cells and neural circuits by genetically engineering them to respond to light. By using optogenetic tools, epilepsy researchers have made breakthroughs in our understanding of seizures and the development of epilepsy. Recent studies have also highlighted a potential new therapeutic avenue. Furthermore, by combining optogenetics with whole brain imaging, seizure circuits can now be monitored in their entirety and manipulated with cell type specificity.
Acknowledgments
This work was supported by an NIH/NIBIB R00 Award (R00EB008738), an Okawa Foundation Research Grant Award, an NIH Director’s New Innovator Award (DP2OD007265), an NSF CAREER Award (1056008), an Alfred P. Sloan Research Fellowship, a Stanford Bio-X Interdisciplinary Initiatives Seed Grant Program, and NIH/NINDS R01 Awards (R01NS087159,R01NS091461), and NIH/NIA Award (RF1AG047666).
Footnotes
Author Contributions
MC, BAD and JHL together researched, authored and edited the manuscript.
Conflict of Interest Statement
The authors declare that they have no competing interests.
References
- Alfonsa H, Merricks EM, Codadu NK, Cunningham MO, Deisseroth K, Racca C, Trevelyan AJ. The Contribution of Raised Intraneuronal Chloride to Epileptic Network Activity. J Neurosci. 2015;35:7715–7726. doi: 10.1523/JNEUROSCI.4105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic Inhibition? How Is it Modified in Epilepsy? Epilepsia. 2000;41:S90–S95. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brodie MJ. Antiepileptic drug therapy the story so far. Seizure. 2010;19:650–655. doi: 10.1016/j.seizure.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin Ja, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Baumgartner J, Seo JH, Korostenskaja M, Lee KH. Bilateral intracranial EEG with corpus callosotomy may uncover seizure focus in nonlocalizing focal epilepsy. Seizure. 2015;24:63–69. doi: 10.1016/j.seizure.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Chiang C-C, Ladas TP, Gonzalez-Reyes LE, Durand DM. Seizure suppression by high frequency optogenetic stimulation using in vitro and in vivo animal models of epilepsy. Brain Stimul. 7:890–899. doi: 10.1016/j.brs.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy M, Dubé CM, Ehrengruber M, Baram TZ. Inflammatory processes, febrile seizures, and subsequent epileptogenesis. Epilepsy Curr. 2014a;14:15–22. doi: 10.5698/1535-7511-14.s2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, Hasso AN, Obenaus A, Baram TZ. A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J Neurosci. 2014b;34:8672–8684. doi: 10.1523/JNEUROSCI.4806-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MT, McBain CJ. The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a “slow” receptor? Curr Opin Neurobiol. 2014;26:15–21. doi: 10.1016/j.conb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, Cho J-H, Leung A, Savvidis G, Ahn S, Moon M, Lee PKJ, Han JJ, Azimi N, Kim K-S, Bolshakov VY, Chung S. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang a, Kopell N, Buckner RL, Graybiel a M, Moore CI, Boyden ES. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol. 2011;105:1393–1405. doi: 10.1152/jn.00828.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy BA, Choy M, Chuapoco MR, Madsen M, Lee JH. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy BA, Choy M, Riegler J, Wells JA, Anthony DC, Scott RC, Lythgoe MF. Imaging seizure-induced inflammation using an antibody targeted iron oxide contrast agent. Neuroimage. 2012;60:1149–1155. doi: 10.1016/j.neuroimage.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Ellender TJ, Raimondo JV, Irkle A, Lamsa KP, Akerman CJ. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J Neurosci. 2014;34:15208–15222. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Blumenfeld H. Targeting Neuronal Networks with Combined Drug and Stimulation Paradigms Guided by Neuroimaging to Treat Brain Disorders. Neuroscientist. 2015;21:460–474. doi: 10.1177/1073858415592377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10:261–270. doi: 10.1038/nrneurol.2014.59. [DOI] [PubMed] [Google Scholar]
- Furman M, Zhan Q, McCafferty C, Lerner BA, Motelow JE, Meng J, Ma C, Buchanan GF, Witten IB, Deisseroth K, Cardin JA, Blumenfeld H. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: Effects on cortical physiology. Epilepsia. 2015;56:e198–e202. doi: 10.1111/epi.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachello CNG, Baines RA. Inappropriate Neural Activity during a Sensitive Period in Embryogenesis Results in Persistent Seizure-like Behavior. Curr Biol. 2015;25:2964–2968. doi: 10.1016/j.cub.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Henderson KW, Gupta J, Tagliatela S, Litvina E, Zheng X, Van Zandt MA, Woods N, Grund E, Lin D, Royston S, Yanagawa Y, Aaron GB, Naegele JR. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J Neurosci. 2014;34:13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashima M, Kinoshita H, Yamaguchi N, Koshino Y. Activation of GABAergic function necessary for afterdischarge generation in rat hippocampal slices. Neurosci Lett. 1996;207:101–104. doi: 10.1016/0304-3940(96)12496-4. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed Chloride Homeostasis and GABAergic Signaling in Human Temporal Lobe Epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Desai M, Knoblich U, Bernstein J, Henninger M, Graybiel AM, Boyden ES, Buckner RL, Moore CI. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J Neurosci. 2011;31:15086–15091. doi: 10.1523/JNEUROSCI.0007-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MS, Jacobs MP, Lowenstein DH. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50:579–582. doi: 10.1111/j.1528-1167.2008.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-G, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2012;32:1188–1206. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej A, Lippert M, Angenstein F, Neubert J, Pethe A, Grosser OS, Amthauer H, Schroeder UH, Reymann KG, Scheich H, Ohl FW, Goldschmidt J. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. Neuroimage. 2014;103:171–180. doi: 10.1016/j.neuroimage.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Bui A, Lew S, Oijala M, Soltesz I. In vivo evaluation of the dentate gate theory in epilepsy. J Physiol. 2015;593:2379–2388. doi: 10.1113/JP270056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. ENEURO.0005-0014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros L, Eelkman Rooda OHJ, Spanke JK, Alva P, van Dongen MN, Karapatis A, Tolner EA, Strydis C, Davey N, Winkelman BHJ, Negrello M, Serdijn WA, Steuber V, van den Maagdenberg AMJM, De Zeeuw CI, Hoebeek FE. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol. 2015;77:1027–1049. doi: 10.1002/ana.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y. Untangling GABAergic wiring in the cortical microcircuit. Curr Opin Neurobiol. 2014;26:7–14. doi: 10.1016/j.conb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Ladas TP, Chiang C-C, Gonzalez-Reyes LE, Nowak T, Durand DM. Seizure reduction through interneuron-mediated entrainment using low frequency optical stimulation. Exp Neurol. 2015;269:120–132. doi: 10.1016/j.expneurol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global Optogenetic Activation of Inhibitory Interneurons during Epileptiform Activity. J Neurosci. 2014;34:3364–3377. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim D-S, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L-WS. Hippocampal electrical activity following local tetanization. I. Afterdischarges. Brain Res. 1987;419:173–187. doi: 10.1016/0006-8993(87)90581-6. [DOI] [PubMed] [Google Scholar]
- Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, Trauner D, Isacoff EY. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis. 2012;47:358–366. doi: 10.1016/j.nbd.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lee HJ, Weitz AJ, Fang Z, Lin P, Choy M, Fisher R, Pinskiy V, Tolpygo A, Mitra P, Schiff N, Lee JH. Frequency-selective control of cortical and subcortical networks by central thalamus. Elife. 2015:4. doi: 10.7554/eLife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhong C, Wang L, Wei P, He W, Huang K, Zhang Y, Zhan Y, Feng G, Wang L. Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat Commun. 2016;7:10962. doi: 10.1038/ncomms10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiuk K, Kontula L, Pitkänen A. cDNA profiling of epileptogenesis in the rat brain. Eur J Neurosci. 2003;17:271–279. doi: 10.1046/j.1460-9568.2003.02461.x. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg. 1995;82:220–227. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O, Deisseroth K. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RNS, Liu G, Gummadavelli A, Zayyad Z, Lee HS, Chu V, Andrews JP, Englot DJ, Herman P, Sanganahalli BG, Hyder F, Blumenfeld H. Decreased Subcortical Cholinergic Arousal in Focal Seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen J, Halonen T, Koivisto E, Pitkänen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- Osawa S-I, Iwasaki M, Hosaka R, Matsuzaka Y, Tomita H, Ishizuka T, Sugano E, Okumura E, Yawo H, Nakasato N, Tominaga T, Mushiake H. Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS One. 2013;8:e60928. doi: 10.1371/journal.pone.0060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Kaila K, Kullmann DM, Miles R. Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions? J Physiol. 2013;591:765–774. doi: 10.1113/jphysiol.2012.237958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Rovner LI. Electrode placement and kindling-induced experimental epilepsy. Exp Neurol. 1978;58:335–346. doi: 10.1016/0014-4886(78)90145-0. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Schwartzkroin PA, Moshé SL, Mareš P, Kubová H. Electrical Stimulation-Induced Models of Seizures. Models of Seizures and Epilepsy. 2006:153–159. [Google Scholar]
- Putnam TJ, Merritt HH. Experimental determination of the anticonvulsant properties of some phenyl derivatives. Science. 1937;85:525–526. doi: 10.1126/science.85.2213.525. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Stark E, Sjulson L, Buzsáki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol. 2014;26:88–95. doi: 10.1016/j.conb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessolo M, Marcon I, Bovetti S, Losi G, Cammarota M, Ratto GM, Fellin T, Carmignoto G. Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J Neurosci. 2015;35:9544–9557. doi: 10.1523/JNEUROSCI.5117-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol. 2015;77:541–546. doi: 10.1002/ana.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon SD. Drug treatment of epilepsy in the century of the ILAE: the first 50 years, 1909–1958. Epilepsia 50 Suppl. 2009a;3:69–92. doi: 10.1111/j.1528-1167.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Shorvon SD. Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959–2009. Epilepsia. 2009b;50(Suppl 3):93–130. doi: 10.1111/j.1528-1167.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- Soper C, Wicker E, Kulick CV, N’Gouemo P, Forcelli PA. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol Dis. 2015;87:102–115. doi: 10.1016/j.nbd.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel E. Quantitative determination of reactivity by electric stimulation of the brain with the skull intact. J Lab Clin Med. 1937;22:1274–1276. [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Chan AM, Ahmed OJ, Rao VR, Gradinaru V, Ramakrishnan C, Deisseroth K, Majewska AK, Cash SS. Optogenetic Delay of Status Epilepticus Onset in an In Vivo Rodent Epilepsy Model Bazhenov M, ed. PLoS One. 2013;8:e62013. doi: 10.1371/journal.pone.0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Bertram EH. Temporal lobe epilepsy. Handb Clin Neurol. 2012;107:225–240. doi: 10.1016/B978-0-444-52898-8.00014-8. [DOI] [PubMed] [Google Scholar]
- Tønnesen J, Sørensen AT, Deisseroth K, Lundberg C, Kokaia M. Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A. 2009;106:12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda I, Fujita S, Thamattoor AK, Buckmaster PS. Unit Activity of Hippocampal Interneurons before Spontaneous Seizures in an Animal Model of Temporal Lobe Epilepsy. J Neurosci. 2015;35:6600–6618. doi: 10.1523/JNEUROSCI.4786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Muldoon SF, Merricks EM, Racca C, Staley KJ. The role of inhibition in epileptic networks. J Clin Neurophysiol. 2015;32:227–234. doi: 10.1097/WNP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FB, Truccolo W, Wang J, Nurmikko AV. Spatiotemporal dynamics of optogenetically induced and spontaneous seizure transitions in primary generalized epilepsy. J Neurophysiol. 2015;113:2321–2341. doi: 10.1152/jn.01040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz AJ, Fang Z, Lee HJ, Fisher RS, Smith WC, Choy M, Liu J, Lin P, Rosenberg M, Lee JH. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage. 2015;107:229–241. doi: 10.1016/j.neuroimage.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S, Nolan SJ, Cotton J, Gandhi S, Weston J, Sudan A, Ramirez R, Newton R. Surgery for epilepsy. Cochrane database Syst Rev. 2015;7:CD010541. doi: 10.1002/14651858.CD010541.pub2. [DOI] [PubMed] [Google Scholar]
- Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, Hashemi KS, Walker MC, Schorge S, Kullmann DM. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4:161ra152. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophysiol. 2015;113:1616–1630. doi: 10.1152/jn.00841.2014. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]