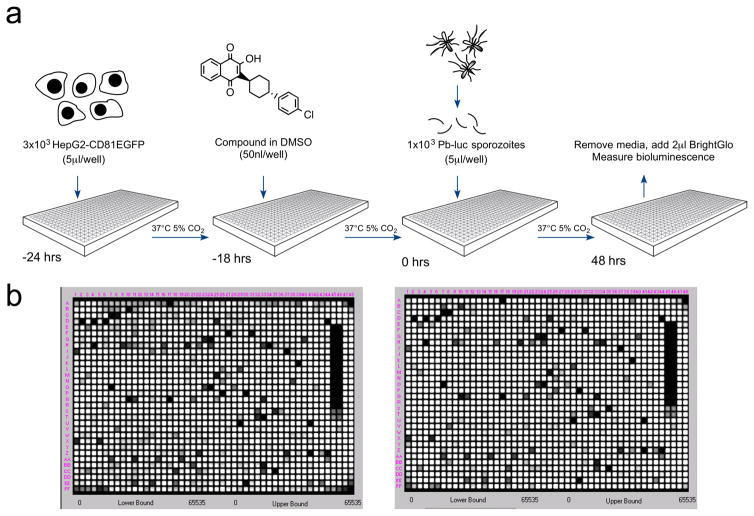
Figure 1. A luciferase-based high-throughput screening assay to identify malaria exoerythrocytic-stage inhibitors.
a) Assay workflow. 24 hours prior to infection, 3×103 HepG2-A16-CD81EGFP cells in 5 μl media were added to wells in a 1536-well assay plate. One to four hours later, 50 nl of compound dissolved in DMSO were added to the wells. At the time of infection, Pb-Luc sporozoites were freshly prepared from infected A. stephensi mosquitoes and diluted to a concentration of 1×103 in 5 μl media per well. After 48 hours, Pb-Luc growth within hepatocytes was measured by bioluminescence.
b) As a proof of concept, we screened two plates containing 2,816 natural compounds (GNF) in replicate. One set of replicates is shown here. The average Z factor for these plates was 0.82.