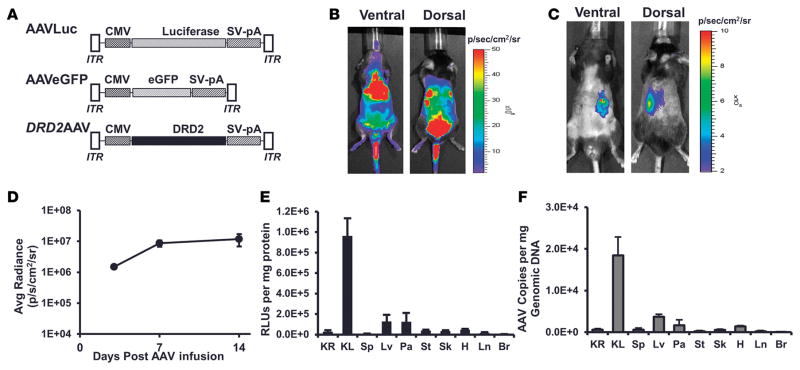
Figure 1. Retrograde ureteral infusion of adeno-associated virus vectors provides renal-specific gene expression.
(A) Schematic representation of adeno-associated virus (AAV) vectors AAVLuc, AAVEGFP, and DRD2 AAV carrying the firefly luciferase cDNA, EGFP, or human dopamine receptor D2 (DRD2) cDNA driven by the CMV promoter. AAV-inverted terminal repeats (ITR) and SV40 polyadenylation signal (SV-pA) are also indicated. Mice were infused (B) systemically via the jugular vein or (C) retrogradely via the left ureter with ACMVLuc vector (1 × 1011 viral genome particles) carrying firefly luciferase under the control of CMV. Shown are representative bioluminescence images of the mouse in ventral and dorsal positions acquired on day 14 following vector administration. (D) Maximum and stable bioluminescence was achieved 14 days after injection. (E) Fourteen days following vector administration, the mice were euthanized and a panel of tissues (KR, right kidney; KL, left kidney; Sp, spleen; Lv, liver; Pa, pancreas; St, stomach; Sk, skeletal muscle; H, heart; Ln, lung; Br, brain) was collected. Luciferase activity in the indicated organs was determined using the in vitro luciferase assay kit (Promega Corporation) on protein extracts prepared as per the manufacturer’s guidelines. Luciferase activities are reported as RLU per mg protein. (F) AAV vector genome copy number in the indicated organs was determined by real-time quantitative PCR. n = 3/group.