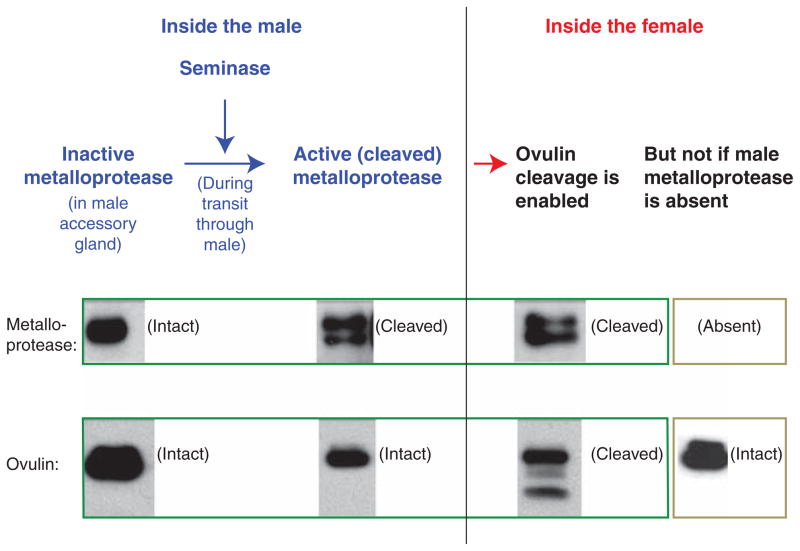
Figure 1.
The molecular cascade that governs the cleavage of the seminal protein ovulin. Biochemical studies show that ovulin cleavage requires activation of a male metalloprotease during transit through the male during mating (Park and Wolfner 1995; Ravi Ram et al. 2006; LaFlamme et al. 2012, 2014). Ovulin and the two proteases that are needed to cleave it (seminase and the metalloprotease CG11864) are all made in the male’s accessory gland but are stored uncleaved in this tissue. During transit through the male, the metalloprotease is activated by cleavage that is initiated by the serine protease “seminase.” Although this metalloprotease is essential for ovulin cleavage within mated females, even after being activated, it does not cleave ovulin until all the proteins have entered females. (Western blot panels from Ravi Ram et al. 2006; reprinted, with permission, from the authors.)