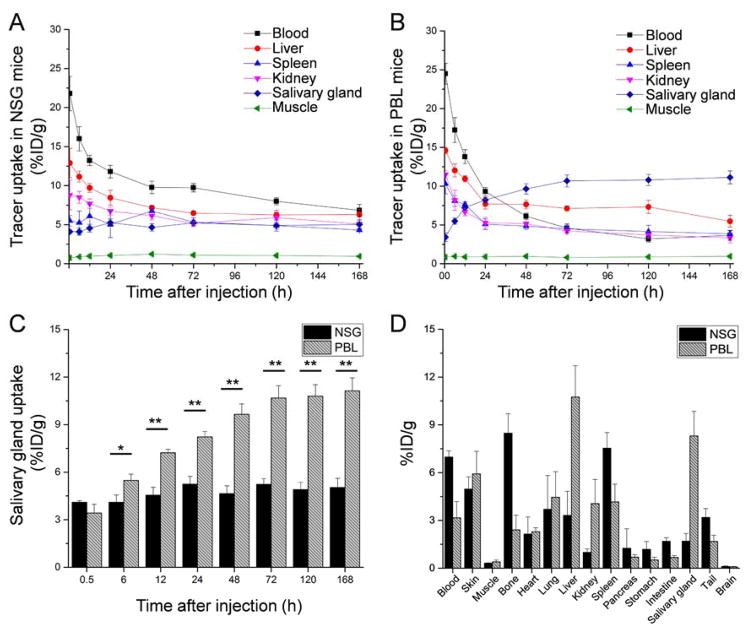
FIGURE 5.
Quantitative analysis of PET data and biodistribution of 89Zr-Df-Pembrolizumab in the PBL mouse model reconstituted with human peripheral blood mononuclear cells (PBMCs) and normal NSG mice. (A) PET ROI analysis is shown as time-activity curves of the blood, liver, spleen, kidneys, and muscle after intravenous injection of 89Zr-Df-Pembrolizumab in NSG mouse model (n=4). (B) PET ROI analysis is shown as time-activity curves of the blood, liver, spleen, kidneys, and muscle after intravenous injection of 89Zr-Df-Pembrolizumab in PBL mice (n=4). (C) Comparison of salivary gland uptake of 89Zr-Df-Pembrolizumab between NSG and PBL mice (n=4). (D) Biodistribution of 89Zr-Df-Pembrolizumab in the blood, organs, and tissues of NSG and PBL mice at 168 h post-injection (n=4). *p<0.05, **p<0.01.