Hematopoietic stem/progenitor cells (HSPCs) are mobilized from bone marrow (BM) into peripheral blood (PB) in steady state conditions in a circadian rhythm-dependent manner as well as in pathological processes related to tissue/organ injury and inflammation. The number of HSPCs circulating in PB is also enhanced by administration of certain drugs, such as granulocyte colony-stimulating factor (G-CSF) or a CXCR4 receptor antagonist (AMD3100). The pharmacologically induced mobilization by G-CSF or AMD3100 is a strategy currently employed in the clinic to obtain HSPCs from mobilized PB for hematopoietic transplantation.1, 2, 3, 4, 5
Evidence has accumulated that elements of innate immunity, such as the complement cascade (ComC), neutrophils, monocytes/macrophages, and naturally occurring IgM antibodies, play a crucial role in mobilization of HSPCs into PB, which can be considered as a response to pro-inflammatory stimuli.2, 6 Toll-like receptors (TLRs) that play a key role in the innate immune system and are expressed by granulocytes and macrophages have been reported to be engaged in crosstalk with complement C3a and C5a receptors and thus are important modulators of inflammatory responses in vivo. This crosstalk reportedly augments ComC-mediated synthesis of several pro-inflammatory cytokines.7, 8
In general, TLRs recognize structurally conserved pathogen-associated molecular pattern molecules (PAMPs), such as endotoxin or liposaccharide (LPS), which are derived from invading microbes. However, at the same time they also recognize certain endogenous ligands belonging to the family of danger-associated molecular pattern molecules (DAMPs), such as extracellular ATP, fibrinogen, heat shock proteins, high mobility group box 1 protein (HMGB1), extracellular matrix components, and self DNA produced by activated cells in the organism.9 In mice, 11 TLRs (TLR1–11) have been described, and all of them except TLR3 use MyD88 as an adapter signaling protein.9 It has been reported that TLRs are also expressed by HSPCs and directly regulate some of their biological functions.10 As recently proposed, G-CSF regulates hematopoietic stem cell activity, in part, through activation of TLR signaling.11
For many years our group has pursued the role of the ComC and other elements of innate immunity in the mobilization of HSPCs. As has been reported, the ComC is activated in BM after pharmacological mobilization by G-CSF or AMD3100.2, 6 On the other hand, the ComC is also strongly activated by certain PAMPs, including LPS and zymosan. Thus, mobilization of HSPCs can be considered as a response of the BM microenvironment and stem cell niches to inflammation and tissue/organ injury.2 We also recently found that heme oxygenase 1 (HO-1) is a negative regulator of ComC activation and inhibits cell migration and mobilization of HSPCs.12, 13
Since TLRs are expressed by both HSPCs and accessory cells that promote the mobilization process (e.g., granulocytes and macrophages) as well as endothelial cells lining the BM sinusoids, we became interested in the role of TLRs in the egress of HSPCs from BM into PB. To address this question, we performed mobilization studies employing G-CSF, AMD3100, LPS, and zymosan in MyD88 mice that lack the adaptor signaling protein associated with all TLRs except TLR3. Furthermore, to address the role of TLR3 in MyD88-independent signaling, we also employed TLR3-KO mice in our mobilization studies. Supplementary Figure 1 shows that, under steady-state conditions, MyD88-KO animals have i) PB counts, ii) erythropoietic parameters, ii) the number of HSCs, and iv) the number of clonogenic CFU-GM, BFU-E, and CFU-Meg progenitors in BM that are similar to WT control mice. Similarly, we did not observe any abnormalities in the evaluated hematopoietic parameters under steady-state conditions in TLR3-KO animals (data not shown).
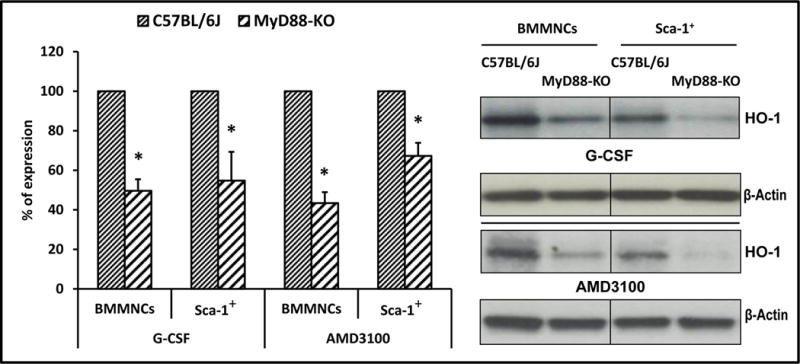
In our studies, MyD88-KO, TLR3-KO, and WT mice were mobilized by G-CSF (100 μg/kg/daily/6days), AMD3100 (5 mg/kg), LPS (200 ng/mice) or zymosan (0.5 mg/mice). Mobilization efficiency in response to mobilizing agents was evaluated in MyD88-KO, TLR3-KO, and WT control animals by determining the number of i) circulating white blood cells (WBCs), ii) Sca-1+c-kit+Lin− (SKL) cells, iii) Sca-1+Lin−CD45+ HSCs, and iv) clonogenic CFU-GM in the PB of mobilized animals. We found that MyD88-KO mice (Figure 1) as well as TLR3-KO mice (Supplementary Figure 2) are more easily mobilized by G-CSF and AMD3100 than WT littermates. Moreover, the enhanced mobilization status of MyD88-KO mice and TLR3-KO mice corresponded with low activation of HO-1, as measured at the mRNA and protein levels in BMMNCs and SKL cells isolated from mutant animals, compared with WT control mice (Figure 2 and Supplementary Figure 2).
Figure 1.
MyD88-KO mice are easily mobilized by G-CSF and AMD3100. MyD88-deficient and wild type mice (as control) were treated with G-CSF (for 6 days at 100 μg/kg per day, subcutaneous injection, upper panel) and AMD3100 (single dose at 5 μg/kg, intraperitoneal injection, lower panel). Experimental mice were sacrificed 6 h after the last G-CSF injection and 1 h after AMD3100 mobilization, and the numbers of WBCs, SKL (Sca-1+ c-kit+ Lin−) cells, HSCs (Sca-1+ CD45+ Lin−) and CFU-GM clonogenic progenitors from PB were evaluated. Results from two separate experiments are pooled together (n = 8 mice per group). *P < 0.05
Figure 2.
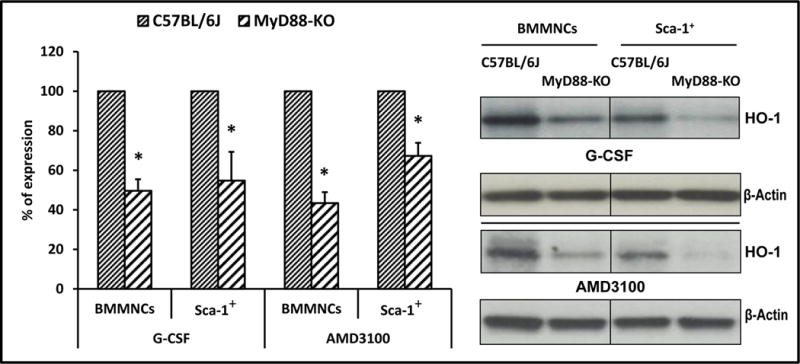
The level of heme oxygenase 1 (HO-1) was evaluated in BMMNCs, and sorted Sca-1+ cells collected from WT and MyD88-KO mice were stimulated with G-CSF (140 ng/ml) or AMD3100 (140 ng/ml). Panel left. Expression of HO-1 at the mRNA level by real-time PCR. Results from three independent experiments are pooled together and shown as the percentage of expression in WT mice. *P ≤ 0.05. Panel right. Expression of HO-1 by western blotting in BMMNCs and Sca-1+ cells purified from WT and MyD88-KO mice. As shown here, the cells from MyD88-KO mice show downregulation of HO-1 compared with WT-derived cells. The experiment was carried out twice with similar results, and a representative blot is shown.
Mobilization after administration of G-CSF or AMD3100 in the context of TLR signaling may depend on the crosstalk between activated ComC receptors and TLRs and could be potentiated by direct stimulation of TLRs by certain endogenous DAMP molecules released from activated BM cells (e.g., heat shock proteins, HMGB1, extracellular matrix components, and self DNA).9 To our surprise, however, we found that expression of TLRs is not obligatory to augmenting mobilization of HSPCs, as MyD88-KO and TLR3-KO animals exhibited enhanced, not decreased, egress of HSPCs into PB in our hands.
Next, we employed PAMPs such as LPS and zymosan, which are potent ligands for TLRs. Specifically, while LPS is a potent activator of TLR4, zymosan preferentially activates TLR2. As mentioned above, both TLR2 and TLR4 depend on the MyD88 adaptor protein for proper signaling. As with G-CSF and AMD3100, we again observed that MyD88-KO mice are easily mobilized by administration of LPS or zymosan compared with WT animals and that this enhanced mobilization correlated again with lower activation of HO-1 in BMMNCs and SKL cells (Supplementary Figure 3). Interestingly, sine TLR2 and TLR4 are classical receptors for zymosan and LPS respectively, our data indicate involvement of MyD88-independent signaling pathways in these cells.
The enhanced mobilization state in TLR signaling-deficient mice could be the result of intrinsic TLR-mediated properties of hematopoietic cells or could depend on a TLR defect in non-hematopoietic BM cells (e.g., stroma or endothelium). To address this question, we created irradiation chimeras by transplanting WT mice with BM from MyD88-KO mice and, vice versa, by reconstituting MyD88 mice with BM from WT animals. To track the chimerism status of transplanted mice, we used matched CD45.1 and CD45.2 murine congenic strains for cross-transplantation, and 3 months after transplantation, chimerism was demonstrated by employing anti-CD45.1 and anti-CD45.2 FACS-based immune identification of the chimeric mice. Both groups of chimeric mice as well as control WT mice transplanted with BM cells from syngeneic WT mice were mobilized by G-CSF or AMD3100, and we evaluated the mobilization efficiency by evaluating the number of WBCs, SKL cells, HSCs, and circulating CFU-GM clonogenic progenitors in PB as described above. Supplementary Figure 4 shows that enhanced mobilization was observed in WT mice that were transplanted with MyD88-KO BM cells and not in MyD88-KO mice transplanted with WT cells. This finding supports the conclusion that enhanced mobilization in TLR signaling-deficient mice depends on lack of TLRs expressed in hematopoietic cells and not on non-hematopoietic cells in the BM microenvironment.
The results presented in this short report are important for several reasons. First, it is evidence that, while TLRs are part of innate immunity and may have crosstalk with C3aR and C5aR, they do not promote, but instead prevent, mobilization of HSPCs into PB.7, 8 This finding is supported by our observation that TLR signaling-deficient mice are easy mobilizers. This negative effect of TLRs on mobilization of HSPCs may somehow balance the mobilization process and may correlate with intracellular expression of HO-1, as TLR-KO mice display a lower level of HO-1 in response to G-CSF, AMD3100, LPS, or zymosan. Since HO-1 is a negative regulator of HSPC trafficking,12, 13 TLRs negatively affect the migration of HSPCs by enhancing HO-1 expression. Our results have been supported by other reports showing that LPS–TLR4 signaling in macrophages, in fact, increases intracellular HO-1 activity.14
An open question remains: what are the receptors responsible for egress of HSPCs, since TLR-KO mice are easily mobilized? It is well known that PAMPs and DAMPs are also recognized by soluble pattern recognition receptors (PRRs), such as mannan-binding lectin (MBL) and ficolins, and that the mannan-binding lectin pathway and PRRs are crucial for activation of the ComC during mobilization.15 Therefore, it is most likely that activation of this pathway leads to enhanced activation of the ComC and, as a consequence, more efficient mobilization of HSPCs. Our results are somewhat supported by a recent report demonstrating enhanced trafficking of HSPCs from BM to spleen in MyD88-KO, TLR2-KO, and TLR4-KO mice.11 Comparing differences in the mobilization of HSPCs in mice treated with antibiotics to eliminate gut commensal microbiota as an endogenous source of the low level of LPS in PB and mutant mice, the authors concluded that trafficking of HSPCs in these mice is TLR-independent. To explain this finding, we propose that MBL and ficolins are most likely the PRRs involved in this process.
In conclusion, these results shed new light on the role of TLRs in the trafficking of HSPCs. We report that TLR signaling involving MyoD88 plays a negative role in egress of HSPCs from BM into PB as TLR signaling enhances expression of HO-1 in hematopoietic cells.14 Moreover, our results from mobilizing irradiation chimeras support the conclusion that this effect of TLRs-associated MyD88 depends on its expression in hematopoietic cells. Based on this finding, we propose that, despite the fact that TLRs have crosstalk with ComC receptors, it is not TLRs but rather soluble PRRs, such as MBL and/or ficolins, that recognize PAMPs and DAMPs and are likely to play a role in triggering activation of the ComC and the mobilization process of HSPCs.
Supplementary Material
Supplementary Figure 1. MyD88-KO deficiency does not affect hematological homeostasis. Bone marrow of WT and MyD88-KO mice was isolated and evaluated for PB white blood cell (A) and red blood cell (B) parameters as well as the number of SKL cells and HSCs in BM (C) and the number of in vitro clonogenic progenitor cells (D). The data represent an average of at least six mice per experimental group.
Supplementary Figure 2. Mobilization of WT and TLR3-KO mice with G-CSF or AMD3100. Animals were treated with G-CSF (for 6 days at 100 μg/kg/dose, subcutaneous injection, Panel A) or AMD3100 (single dose at 5 μg/kg, intraperitoneal injection, Panel B). Mice were sacrificed 6 h after the last G-CSF injection or 1 h after AMD3100 mobilization, and the numbers of WBCs, SKL (Sca-1+ c-kit+ Lin−) cells, HSCs (Sca-1+ CD45+ Lin−), and CFU-GM clonogenic progenitors from PB were evaluated. Results from two separate experiments are pooled together (n = 8 mice per group). *P<0.05. The level of heme oxygenase 1 (HO-1) was evaluated in BMMNCs collected from WT and TLR3-KO mice stimulated with G-CSF (140 ng/ml) or AMD3100 (140 ng/ml), Panel C. Expression of HO-1 at the mRNA level by real-time PCR (Panel C, left). Results from three independent experiments are pooled together and shown as a percentage of expression in WT mice. *p ≤ 0.05. Expression of HO-1 by western blotting in BMMNCs purified from WT and TLR3-KO mice (Panel C, right). The experiment was carried out twice with similar results, and a representative blot is shown.
Supplementary Figure 3. Mobilization of WT and MyD88-KO mice with LPS or zymosan. MyD88-deficient mice are easily mobilized by LPS or zymosan. Experimental mice were treated with LPS (single dose at 200 μg/mouse, panel A) or zymosan (single dose at 0.5 mg/mouse, panel B). Animals were sacrificed 2 h after LPS and 1 h after zymosan mobilization, and the numbers of WBCs, SKL (Sca-1+ c-kit+ Lin−) cells, HSCs (Sca-1+ CD45+ Lin−), and CFU-GM clonogenic progenitors were evaluated from PB. Results from two separate experiments are pooled together (n = 8 mice per group). *P < 0.05. Panel C. The level of heme oxygenase 1 (HO-1) evaluated in BMMNCs and sorted Sca-1+ cells collected from WT and MyD88-KO mice stimulated with LPS (200 ng/ml) or zymosan (50 μg/ml). Expression of HO-1 at the mRNA level according to real-time PCR (Panel C, left). Results from three independent experiments are pooled together and shown as a percentage of the expression in WT mice. *P ≤ 0.05. Expression of HO-1 by western blotting in BMMNCs and Sca-1+ cells purified from WT and MyD88-KO mice (Panel C, right). The experiment was carried out twice with similar results, and a representative blot is shown.
Supplementary Figure 4. Mobilization of radiation chimeras. Irradiated CD45.1 and MyD88-KO (CD45.2) mice were transplanted with MyD88-KO or CD45.1 BMMNCs, respectively (5×106 cells per mouse). The control for group III was CD45.1 mice transplanted with CD45.1 cells. CD45.1 chimeric mice reconstituted with MyD88-KO BM and MyD88-KO chimeric mice transplanted with CD45.1 BM cells were mobilized with G-CSF for 6 days (upper panel) or with one dose of AMD3100 (lower panel). In both cases, mobilization was evaluated by the number of circulating WBCs, SKL cells, and HSCs per microliter of PB by flow cytometry and the number of in vitro CFU-GM clonogenic progenitors per milliliter of PB. Results from two separate experiments are pooled together (n = 8 mice per group). *P < 0.05.
Acknowledgments
This work was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and Harmonia NCN grant: UMO-2014/14/M/NZ3/00475 to MZR.
Footnotes
Conflict-of-Interest Statement
The authors declare no conflicts of interest.
Literature
- 1.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 2.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27:24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, et al. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 7.Song WC. Crosstalk between complement and toll-like receptors. Toxicol Pathol. 2012;40:174–182. doi: 10.1177/0192623311428478. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shcheblyakov DV, Logunov DY, Tukhvatulin AI, Shmarov MM, Naroditsky BS, Gintsburg AL. Toll-Like Receptors (TLRs): The Role in Tumor Progression. Acta Naturae. 2010;2:21–29. [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, et al. G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia. 2014;28:1851–1860. doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamiak M, Moore JB, IV, Zhao J, Abdelbaset-Ismail A, Grubczak K, Borkowska S, et al. Downregulation of heme oxygenase 1 (HO-1) activity in hematopoietic cells enhances their engraftment after transplantation. Cell Transplant. 2015 doi: 10.3727/096368915X688957. e-pub ahead of print 16 October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysoczynski M, Ratajczak J, Pedziwiatr D, Rokosh G, Bolli R, Ratajczak MZ. Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev. 2015;11:110–118. doi: 10.1007/s12015-014-9547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Zhang F, Zhang Z, Peng M, Wang Y, Chen Y. TLR4 signaling-induced heme oxygenase upregulation in the acute lung injury: role in hemorrhagic shock and two-hit induced lung inflammation. Mol Biol Rep. 2013;40:1167–1172. doi: 10.1007/s11033-012-2158-y. [DOI] [PubMed] [Google Scholar]
- 15.Adamiak M, Abdelbaset-Ismail A, Ratajczak J, Ratajczak MZ. A Novel and Pivotal Role of the Mannose-Binding Lectin (MBL) Pathway of Complement Cascade (ComC) Activation in Triggering Mobilization of Hematopoietic Stem/Progenitor Cells (HSPCs). 57th ASH (American Society of Hematology) Annual Meeting and Exposition; Orlando. December 4–8, 2015; (abstract 4301) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. MyD88-KO deficiency does not affect hematological homeostasis. Bone marrow of WT and MyD88-KO mice was isolated and evaluated for PB white blood cell (A) and red blood cell (B) parameters as well as the number of SKL cells and HSCs in BM (C) and the number of in vitro clonogenic progenitor cells (D). The data represent an average of at least six mice per experimental group.
Supplementary Figure 2. Mobilization of WT and TLR3-KO mice with G-CSF or AMD3100. Animals were treated with G-CSF (for 6 days at 100 μg/kg/dose, subcutaneous injection, Panel A) or AMD3100 (single dose at 5 μg/kg, intraperitoneal injection, Panel B). Mice were sacrificed 6 h after the last G-CSF injection or 1 h after AMD3100 mobilization, and the numbers of WBCs, SKL (Sca-1+ c-kit+ Lin−) cells, HSCs (Sca-1+ CD45+ Lin−), and CFU-GM clonogenic progenitors from PB were evaluated. Results from two separate experiments are pooled together (n = 8 mice per group). *P<0.05. The level of heme oxygenase 1 (HO-1) was evaluated in BMMNCs collected from WT and TLR3-KO mice stimulated with G-CSF (140 ng/ml) or AMD3100 (140 ng/ml), Panel C. Expression of HO-1 at the mRNA level by real-time PCR (Panel C, left). Results from three independent experiments are pooled together and shown as a percentage of expression in WT mice. *p ≤ 0.05. Expression of HO-1 by western blotting in BMMNCs purified from WT and TLR3-KO mice (Panel C, right). The experiment was carried out twice with similar results, and a representative blot is shown.
Supplementary Figure 3. Mobilization of WT and MyD88-KO mice with LPS or zymosan. MyD88-deficient mice are easily mobilized by LPS or zymosan. Experimental mice were treated with LPS (single dose at 200 μg/mouse, panel A) or zymosan (single dose at 0.5 mg/mouse, panel B). Animals were sacrificed 2 h after LPS and 1 h after zymosan mobilization, and the numbers of WBCs, SKL (Sca-1+ c-kit+ Lin−) cells, HSCs (Sca-1+ CD45+ Lin−), and CFU-GM clonogenic progenitors were evaluated from PB. Results from two separate experiments are pooled together (n = 8 mice per group). *P < 0.05. Panel C. The level of heme oxygenase 1 (HO-1) evaluated in BMMNCs and sorted Sca-1+ cells collected from WT and MyD88-KO mice stimulated with LPS (200 ng/ml) or zymosan (50 μg/ml). Expression of HO-1 at the mRNA level according to real-time PCR (Panel C, left). Results from three independent experiments are pooled together and shown as a percentage of the expression in WT mice. *P ≤ 0.05. Expression of HO-1 by western blotting in BMMNCs and Sca-1+ cells purified from WT and MyD88-KO mice (Panel C, right). The experiment was carried out twice with similar results, and a representative blot is shown.
Supplementary Figure 4. Mobilization of radiation chimeras. Irradiated CD45.1 and MyD88-KO (CD45.2) mice were transplanted with MyD88-KO or CD45.1 BMMNCs, respectively (5×106 cells per mouse). The control for group III was CD45.1 mice transplanted with CD45.1 cells. CD45.1 chimeric mice reconstituted with MyD88-KO BM and MyD88-KO chimeric mice transplanted with CD45.1 BM cells were mobilized with G-CSF for 6 days (upper panel) or with one dose of AMD3100 (lower panel). In both cases, mobilization was evaluated by the number of circulating WBCs, SKL cells, and HSCs per microliter of PB by flow cytometry and the number of in vitro CFU-GM clonogenic progenitors per milliliter of PB. Results from two separate experiments are pooled together (n = 8 mice per group). *P < 0.05.


