Abstract
Gastric diseases cause considerable worldwide burden. However, the stomach is still poorly understood in terms of the molecular-cellular processes that govern its development and homeostasis. In particular, the complex relationship between the differentiated cell types located within the stomach and the stem and progenitor cells that give rise to them is significantly understudied relative to other organs. In this review, we will highlight the current state of the literature relating to specification of gastric cell lineages from embryogenesis to adulthood. Special emphasis is placed on substantial gaps in knowledge about stomach specification that we think should be tackled to advance the field. For example, it has long been assumed that adult gastric units have a granule-free stem cell that gives rise to all differentiated lineages. Here we will point out that there are also other models that fit all extant data, such as long-lived lineage-committed progenitors that might serve as a source of new cells during homeostasis.
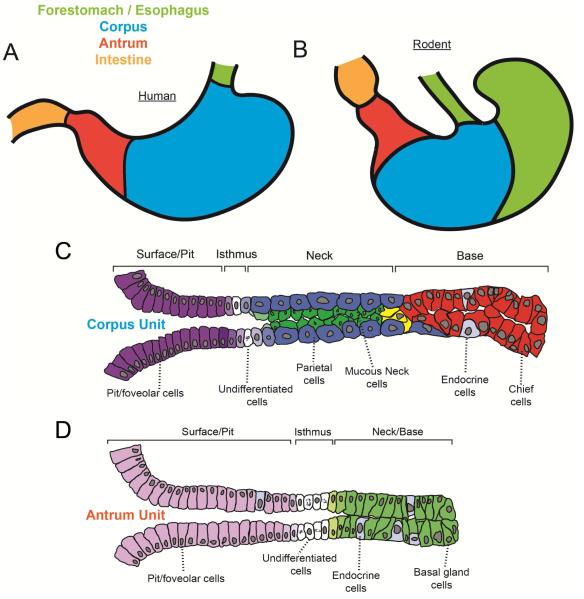
The adult stomach produces acid and enzymes that aid in food digestion and kill microbes, and it regulates delivery of food to the small intestine. The stomach also works remotely via its endocrine cells that send distal signals to help coordinate hunger/satiety and Ca++ homeostasis1. The stomach comprises tissues originating from all three embryonic germ layers including the ectodermally-derived enteric nerves, mesodermally-derived smooth muscle and mesenchymal cells, and the endodermally-derived epithelium lining the lumen of the stomach. In this review, we will largely focus on the processes governing epithelial development and homeostasis. The glandular epithelium in most mammals is arranged into two principal compartments: corpus and antrum (Fig. 1). Both compartments are composed of a single layer of epithelial cells arranged into invaginated units. The principal cellular constituents of corpus units include the surface mucous (pit/foveolar) cells, acid-secreting parietal cells, mucous neck cells, digestive-enzyme secreting (zymogenic) chief cells, endocrine cells, and isthmal cells with undifferentiated features that likely serve as multipotent stem cells. The antral units can contain some chief and parietal cells depending on the species but are primarily composed of pit/foveolar cells on the surface and deep glandular cells that express markers of both mucous neck cells and chief cells (Fig. 1). Scattered throughout corpus and antrum are the rarer endocrine cells, each type named for the predominant hormone they secrete (e.g., gastrin-secreting G-cells of the antrum).
Figure 1. Architecture of the adult stomach and the organization corpus and antral units.
The adult human stomach (A) is entirely composed of glandular epithelium (blue, red) while the adult rodent stomach (B) contains a squamous-epithelium-lined forestomach (green) in addition to a glandular stomach. Adult corpus units (C) contain pit/fovelar cells (purple), isthmal stem cells (white), parietal cells (blue), mucous neck cells (green), endocrine cells (light blue), and chief cells (red). Cells transitioning from neck to chief cells are indicated in yellow. Antral units (D) primarily contain pit/fovelar cells (light purple), proliferative isthmal stem cells (white), basal gland cells (light green) similar to mucous neck cells with a hint of chief cell differentiation, and endocrine cells (grey). Note that up to half of human antral gastric units also contain parietal cells (not depicted).
Understanding cellular development in the normal stomach should help us better understand the origins of gastric cancer, one of the most common causes of cancer death world-wide2. Most gastric cancer is initiated in the setting of chronic infection with the bacterium Helicobacter pylori (H. pylori), which is estimated to infect over half the world’s population3. In addition to increasing risk for gastric cancer, it is also the cause of most ulcers of the stomach and duodenum. Those patients at risk for gastric cancer exhibit a response to infection with H. pylori characterized by an overall loss of specific differentiated cell lineages, a condition known pathologically as chronic atrophic gastritis. Molecular and cellular mechanistic studies have shown that chronic atrophic gastritis is not simply characterized by a chronic inflammatory infiltrate (gastritis) and the loss of acid-secreting parietal cells (oxyntic atrophy) but also by changes in differentiation of the chief cells (metaplasia)4-6. A thorough understanding of the processes that control the specification of cells within the gastric epithelium during development and adult homeostasis could be crucial to deciphering disease etiology, particularly the metaplastic changes that arise after H. pylori infection. However, currently in the stomach, in both the adult and embryonic state, there is rudimentary understanding of the cell lineage relationships. Furthermore, there is also a marked lack of lineage-specific markers and genetic tools for studying development and differentiation. In this review, we will highlight the relatively limited information we have about stomach specification starting with the embryo and continuing through adulthood.
One caveat is that most of the work on mammalian gastric development has been in rodents. Much work has also been done in non-mammalian model organisms like chick. The degree to which human gastric development follows the same rules as rodents – let alone non-mammalian vertebrates -- is not known in most cases. Due to our relatively close ancestry, it is likely most developmental patterns will be similar between humans and these model organisms. However, there are some known differences. For example, the human stomach is lined entirely by glandular units while the rodent stomach contains an additional anatomic compartment known as the forestomach, which is not glandular at all, but, rather, lined with squamous epithelium (Fig. 1). In human stomach, up to half of antral units harbor parietal cells, whereas they are absent from antral units in the rodent7. Also, chief cells in the rodent express gastric intrinsic factor, whereas intrinsic factor is expressed by parietal cells in humans8.
Early Specification
Gastric specification in the mouse begins during gastrulation with derivation of the endodermal germ layer that will eventually seed the epithelial lining of the digestive, respiratory, and urogenital systems. The endoderm germ layer is formed by the ingression of epiblast cells through the primitive streak. As the cells exit the primitive streak, they arrange into a single-layered epithelial sheet on the outside of the embryo (E6-E7.5). This sheet forms pockets at the anterior (future foregut) and posterior (future hindgut) end of the embryo and progressively “zippers” into a complete gut tube. Zippering of the gut tube, mesodermal growth, and embryonic turning transform the endodermal sheet on the outside of the embryo to an internal tube consisting of three major regions – foregut, midgut, and hindgut (E7.5-9)9. Regional and subsequent organ identity is assembled within the naïve, as yet unspecified gut tube through the integration of signaling inputs from mesodermal tissues located apposed to the endoderm and the endodermal progenitors themselves10. One recognizable output of the stage when regional identity is acquired is a pattern of expression of overlapping transcription factor domains that facilitate subsequent organ-specific differentiation programs.
Stomach epithelial progenitors derive from the foregut region of the endoderm, which also gives rise to liver, pancreas, lungs and the luminal GI organs from the pharynx to the anterior duodenum. Signaling pathways and transcription factors that drive specification of pre-gastric endodermal progenitors from other emerging organs within the foregut have not been well characterized11. However, a number of signaling pathways that promote or restrict foregut identity by patterning the anterior/posterior axis of the endoderm are known. Retinoic acid (RA), for example, has a complex spatiotemporal role patterning the anterior-posterior (A-P) axis of the endoderm. During late gastrulation, RA signaling promotes the specification of posterior endodermal fates over anterior endodermal fates particularly at the foregut-midgut boundary12, 13. Subsequently, RA signaling is required to promote the development of a number of foregut tissues. Animals with defective RA signaling have abnormal stomach development, but specific consequence to gastric specification is unclear14. Wnt and FGF signals produced by the mesoderm promote expression of posterior endodermal markers like Cdx2 over anterior endodermal markers15-17. Studies in zebrafish have also shown that BMP signaling drives posterior over anterior endodermal fates18.
Through the study of other endodermal organs, a number of tissues have been shown to produce important signaling molecules to promote foregut organ specification. For example, the dorsal aorta and notochord produce several key signaling molecules involved in dorsal pancreatic specification19, 20. These same tissues could also impact pre-gastric gene expression given the proximity of gastric and dorsal pancreatic progenitors. Ventral tissues, including cardiac mesoderm, could also impact gastric specification from other ventral organs like the liver and lung21. Other signaling pathways like Shh have been implicated in gastric growth through epithelial to mesenchyme signaling, though Shh does not appear to be involved in gastric specification22.
During endodermal specification, a highly conserved core transcription network (including FoxA, Gata, Sox17, and Mixl1 transcription factors) is activated and guides the growth and survival of endodermal cells prior to regionalization23. Expression of these transcription factors in early endoderm is necessary to generate foregut progenitors that give rise to the stomach. As the endoderm regionalizes, a number of transcription factors are expressed either throughout the foregut endoderm or regionally in the pre-gastric domain. Broadly expressed transcription factors like Foxa1/2/3, Gata4/6, Hnf1β, and Sox2 all could play an important role in gastric specification (Fig. 2). For example, the FoxA family is expressed throughout the early endoderm and is important in the development of a number of organs including the liver, pancreas, and intestine24-26. The specific role of this family in the stomach has yet to be determined; however, FoxA’s are known to be involved in promoting expression of Pdx1 in the foregut (Fig. 2). Because Pdx1 is expressed only in the gastric antrum and not the more proximal corpus27, FoxA factors could thus be involved in regionalizing the stomach25.
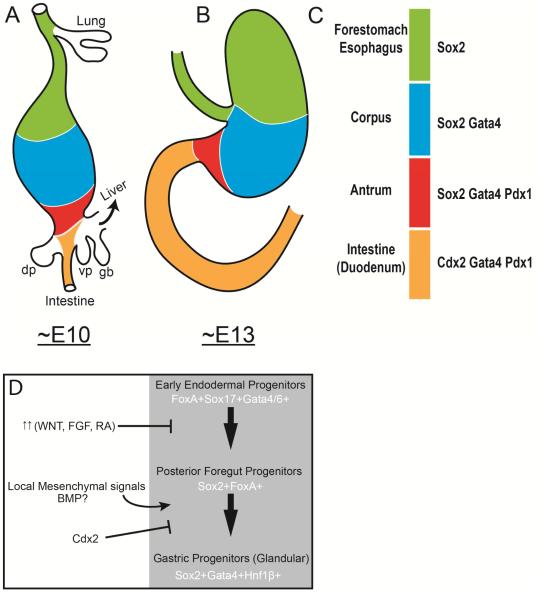
Figure 2. Transcription factor domains in the development gastric region.
Schematic representation of the mouse developing posterior foregut at (A) ~E10 and (B) ~E13. Color codes in (A) and (B) correspond to specific transcription factor signatures in (C). In Green is the future forestomach and esophagus that expresses Sox2 but not other glandular markers like Gata4 and Pdx1. The future corpus (blue) expresses Sox2 and Gata4 but not the more posterior regional markers like Pdx1. The future antrum (red) expresses Sox2, Gata4, and Pdx1 but not the intestinal marker Cdx2. The future anterior small intestine expresses Cdx2, Gata4, and Pdx1 but not the anterior endodermal marker Sox2. The anterior boundary of Gata4 (blue/green border) is expressed in the glandular stomach but not the forestomach (green). (D) Speculative model of glandular stomach specification during development. Based on developmental studies, early foregut progenitors express the important transcription factors of the FoxA family, Sox17, and Gata4/6. Around this time, an appropriate balance of WNT, FGF, and RA signaling is needed to specify the region of the gut that gives rise to gastric progenitors. These pathways actively posteriorize the endoderm –too little or too much signaling could drive the endoderm to more anterior or posterior fate, respectively. Future gastric progenitor need to acquire Sox2 expression and not the intestine determinant Cdx2, which is expressed in more posterior endoderm. Once organ budding begins, local mesenchymal signals are crucial to enforce glandular identity and repress adjacent non-glandular stomach organ fates like esophagus/forestomach and intestine. Potentially these signals act through driving expression of potential gastric specification transcription factors like Gata4 and Hnf1β.
Gata4 and Gata6 are involved in the specification of the extraembryonic endoderm28-30 and are expressed throughout the early definitive endoderm. During endodermal regionalization, both genes are expressed in the foregut. The expression domain of Gata4 is particularly interesting, as its anterior boundary resides at the future forestomach/glandular stomach boundary. Potentially, Gata4 may have an important role in specifying the glandular stomach or specifying the forestomach vs. the glandular stomach (Fig. 2). Consistent with the idea that Gata4 is important for glandular stomach specification, Gata4 null cells do not appear to be able to adopt gastric identity in chimeric embryos when they are competing with wildtype cells31.
Sox2 is broadly expressed throughout the foregut from the most anterior pharyngeal endoderm to the future boundary of gastric antrum and duodenum. Studies wherein expression of Sox2 is reduced in developing endoderm have shown that it helps govern the development of a number of foregut organs including the stomach, esophagus, trachea, and lung32, 33. Such experiments involved hypomorphic animals, so it will be interesting to know what the effects of complete loss of SOX2 from early endoderm might be. Perhaps SOX2 has an even more critical role in anterior foregut and stomach specification than currently thought. The border between Sox2 and Cdx2 expression during development (Fig. 2) resides at the prospective gastrointestinal junction and suggests that Sox2 could define this boundary. Misexpressing Sox2 in Cdx2-postive progenitors in the developing intestine increases expression of gastric-specific differentiation markers34. Interestingly, loss of Cdx2 during early development causes a dramatic transformation of the prospective intestine into Sox2 expressing esophageal-like progenitors and not gastric progenitors35, indicating that SOX2 is not a simple pro-gastric, anti-intestine transcription factor. Indeed, SOX2 levels are high in both adult esophagus and adult stomach36.
Pdx1 is regionally expressed within the posterior foregut in the areas that give rise to the posterior stomach (antrum/pylorus), anterior duodenum, dorsal and ventral pancreatic buds, and proximal extra hepatic biliary system27, 37. Pdx1 expression can be used during development to distinguish antral gastric progenitors (SOX2+GATA4+PDX1+) from corpus progenitors (SOX2+GATA4+PDX1−). Loss of Pdx1 causes aberrant antral stomach progenitors including pyloric defects27 and loss of gastrin-producing endocrine cells38. Hnf1β is expressed in the early endoderm and implicated in stomach specification. Definitive endoderm-specific knockout of Hnf1β alters gene expression within caudal stomach progenitors, including causing loss of Pdx1 and Ihh39. The impact on gastric specification in these knockouts remains unclear, but recent in vitro studies have, intriguingly, implicated Hnf1β in promoting antral stomach specification in organoid culture40.
To date, no specific gene has been shown to have expression restricted only to early gastric progenitors; thus, it remains difficult to examine directly how the stomach is specified from other organs, the way, for example, Cdx1 and Cdx2 have been studied in intestinal specification. Instead, investigators rely on more broadly expressed genes (ie, expressed concomitantly in other organs besides stomach) like Sox2, Gata4 and Pdx1 to identify the factors defining the prospective gastric regions. Further identification of transcripts that may have more restricted or specific expression to gastric progenitors (particularly to the glandular stomach) during early development could lead to the generation of new genetic tools to explore and characterize gastric specification or even to perform stomach-specific epithelial cell gene deletion as intestinal epithelial specific deletion can be driven by Villin-Cre. However, there could be marked improvement in our understanding of stomach specification simply by manipulating gene expression in early endoderm with tools that already exist. For example, signaling pathways and transcription factors suspected of being involved in gastric development could be deleted via crosses to well characterized mouse pedigrees that express Foxa3-, Sox17-, or Shh- Cre41-43.
Summing up all that is currently known and can be inferred from published studies, we have proposed one possible signaling and epistasis model for specification of glandular stomach (Fig. 2).
Mesoderm
Regionalization throughout the luminal gastrointestinal tract depends in large part on epithelial-mesenchymal crosstalk, and the stomach does not seem to be an exception. For example, foundational experiments in chick have shown that placing proventricular (stomach region in chick similar to the mammalian glandular stomach) mesenchyme with gizzard (anterior chicken stomach) or esophageal endoderm induces proventricular gene expression and causes gland development in these normally non-glandular tissues44, 45. Similarly, gizzard or esophageal mesenchyme can suppress proventricular gene expression and gland development in proventricular endoderm and promote squamous fates46. Interestingly, proventricular mesenchyme could not induce proventricular gene expression in intestinal endoderm47; hence, overlying mesoderm can instruct endoderm identity but only within restricted regions. BMP factors have been implicated in promoting proventricular identity48.
While BMP signaling, principally deriving from the mesenchyme, influences gastric epithelilal development, Hedgehog signaling derived from the epithelium influences the mesenchyme. For example, in addition to their early role in foregut growth, Hedgehog (Shh/Ihh) signals are produced by the gastric endoderm to support mesenchymal growth and differentiation, a pattern which is maintained in the adult49, 50. Another example of a factor that originates from the mesenchyme and regulates the epithelium is FGF10, likely via the receptor FGFR2B51. FGF10 promotes epithelial proliferation and gland development51, 52. Though it may not be required for adult homeostasis, it has been shown to inhibit parietal and chief cell differentiation in favor of the mucous neck cell type53.
In addition to the themes of epithelial Hedgehog and mesenchymal BMP signaling that occur throughout the gastrointestinal tract, there have been some descriptions of signals more specific to gastric development vs. other regions. For example, Barx1 is a transcription factor that is restricted to the prospective esophageal and gastric mesoderm. Barx1 null mice have significantly altered stomach morphology with disrupted patterning of the stomach. The stomach-intestinal boundary is disturbed such that ectopic CDX2+ intestinal epithelial cells can be found in the posterior stomach54, 55. In addition to the disrupted inter-organ patterning, the division of intra-stomach domains is altered. For example, H+/K+-ATPase expressing cells are seen intermingled with PDX1+ cells, which in mice are normally exclusive to the corpus and antrum, respectively. Bapx1 (Nkx3-2), Nkx2-5, Gata3, Six2, Nr2f2 (COUP-TF II), and Sox9 are other known transcription factors expressed in the posterior stomach mesenchyme and involved in specifying the pylorus56-58. In the absence of those transcription factors, there is aberrant neuromuscular regulation of the pyloric sphincter, which in humans can manifest as the relatively common condition known as pyloric stenosis58, 59.
Cell Lineage Specification
In between the stage of endodermal specification and the stage of specific cell lineage commitment in the stomach, the gastric epithelium remains a simple epithelium with no obvious differentiation. Around E14.5 to 16.5, markers representative of cell types like endocrine, parietal, and chief cells begin to be expressed, and small glands begin to invaginate into the mesenchyme from the simple epithelium lining the lumen52. Between E16.5 and 2 weeks of postnatal development, most of the major cell types arise within the stomach, and the glandular stomach mostly becomes organized into its adult form. However, the murine stomach doesn’t reach adult organization with full chief cell and endocrine cell lineage specification until 6-8 weeks postnatally60. For most cell lineages in the stomach we have a poor understanding of pathways and factors involved in their specification and the progenitors from which they directly derive. For example – and this is truly remarkable when contrasted to the state of our understanding in the intestines -- there is no specific factor that is known to be necessary or sufficient for specification of chief, parietal, pit, mucous neck, or isthmal cells. The markers used in gastric biology represent the terminal differentiation of those cells (e.g.: Atp4b, Tff1, Pgc, Gif). The lack of this basic specification knowledge greatly hinders deciphering the molecular mechanisms underlying how gastric disease causes the loss or increase of any particular cell lineage. The developmental sequence between gastric epithelial progenitors in an adult gastric unit and the differentiated progeny that arise continuously throughout life is also unknown. It is entirely possible that all the mature cell types are specified from a single multipotent progenitor that persists throughout life61, or there might, in turn, be numerous long-lived lineage-restricted progenitors62, 63 (Fig 3, 4 and see detailed discussion below)
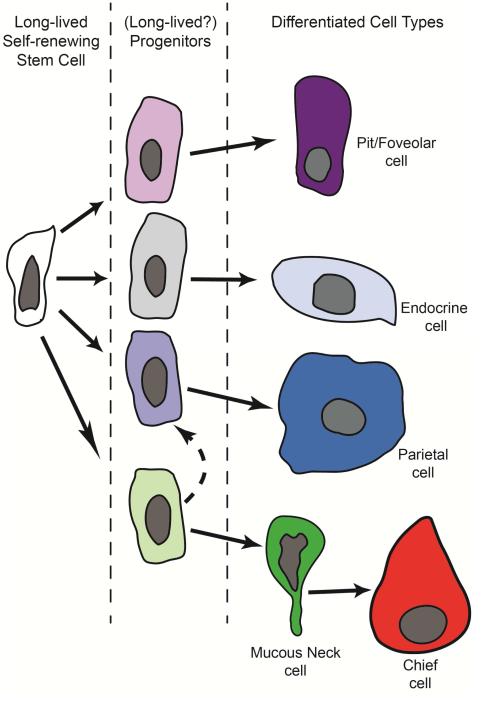
Figure 3. Putative lineage tree of the adult corpus stem cell.
Based on Karam and Leblond’s labeling and ultrastructural studies, the isthmus contains a granule-free stem cell that enters the cell cycle to give rise to progenitors that migrate up and down the corpus unit. Cells that migrate up the unit adopt a pre-pit phenotype (light purple) and eventually turn into mature pit cells (purple). Cells that migrate down the unit appear to adopt a pre-neck (light green), pre-parietal (light blue) or pre-endocrine/endocrine phenotype (grey). Neck cells (green) appear to undergo a further transition at the bottom of the unit and eventually become transitional cells with both neck and chief cell characteristics, and finally fully mature chief cells. It is clear that the granule-free cell is long-lived and self-renewing, but each of the progenitors committed to more specific lineage(s) might also be long-lived and self-renewing as well.
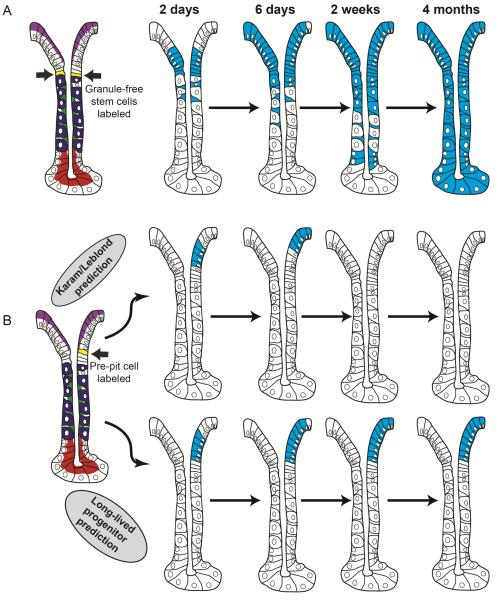
Figure 4. Potential behavior of the adult stem cell and lineage-committed progenitors in the adult corpus.
(A) If Karam and Leblond’s prediction that there is a single adult stem cell in the corpus holds true, then labeling that cell will eventually result in the long-term maintenance of label as well as labeling of all corpus cell types. (B) It remains possible that the corpus contains long-lived lineage restricted progenitors as well. Such cells would have early characteristics of pit cells or neck cells; they would be self-renewing and long-lived but give rise only to differentiated pit or neck/chief cells, respectively. The only studies performed to date to understand how stem cells behave in the stomach: labeled-nucleotide pulse-chase experiments performed by Karam and Leblond et al. would not be able to distinguish between the two possibilities (ie, a long-lived multipotent stem cell vs. long-lived committed progenitors). Lineage tracing experiments with an appropriate promoter (eg, like Lgr5 in the intestine) should be able to distinguish how stem cell hierarchies are arranged. Examples of different lineage tracing patterns with hypothetical, appropriate promoters are shown. If a promoter that is pit-cell-lineage specific could be induced and traced, then the Karam/Leblond model (all cells rapidly arise from a long-lived, self-renewing, multipotent stem cell) would result in temporary labeling of the pit lineage with eventual loss of the label, because the stem cell would not be labeled, and pit cell progenitors are not long-lived. However, if long-lived lineage restricted progenitors exist (contrary to Karam/Leblond), then labeling the pre-pit cell will result in maintenance of the label throughout the pit cell lineage, because the pre-pit cells will self-renew and not die, but they will continue to label all their progeny. Similar predictions would hold to other cell lineages in the corpus.
The only stomach lineages with known genetic determinants and known progenitor markers are endocrine cells, which are controlled by the master regulators Ascl164 and Ngn365, 66. Ngn3 marks endocrine progenitor cells but not mature forms. Ngn3 null embryos lack gastrin, somatostatin, and glucagon endocrine cell types with largely reduced census of serotonin-positive cells, but ECL and ghrelin populations are still present65, 66. Ascl1 null embryos wholly lack gastrin, somatostatin, and glucagon secreting endocrine cell types (the former two missing from their usual niches in the stomach), and gastric serotonin and ghrelin endocrine cells are decreased in number. Ascl1 null embryos die before ECL cells emerge developmentally64; however, it was noted that the vast majority of chromogranin A-positive cells (chromogranin A is a general marker of endocrine cells) are missing in Ascl1 null embryos, and ECL cells represent the majority of chromogranin-positive cells in the corpus. Thus, if Ascl1 is required for all chromogranin A-positive cells to emerge, a conditional deletion in the adult might also reveal that ECL cells are Ascl1-dependent, though this can only be speculated with current data.
Taken together, the data show that Ascl1 and Ngn3 are each required to specify gastrin, somatostatin, and glucagon positive endocrine cells. The eventual emergence of ECL cells may be dependent on Ascl1 but not Ngn3. Serotonin-positive endocrine cells in the antrum are also largely lost in Ascl1 and Ngn3 mutants. A recent study revealed that serotonin-positive cells in the corpus are bone marrow-derived, mucosal-associated mast cells and not descendent from endodermal progenitors, an Ngn3 lineage, or epithelial cells at all67. If corpus serotonin-positive cells are not derived from the endoderm, those cells likely account for the presence of non-antral serotonin-positive cells in Ascl1 mutants mentioned above. It is unclear how specific mature endocrine cell types arise from endocrine-committed progenitors during embryogenesis and adult homeostasis; however, endocrine specification in the pancreas and intestine may serve as an illustrative model68, 69.Endocrine-committed progenitors derived from Ascl1- or Ngn3-positive populations differentiate into individual endocrine cell types depending on the specific downstream transcription program that is enacted. There is indication that similar programs exist in the stomach, as mice null for various transcription factors have defects in specific endocrine lineages. For example, Pdx1, Nkx6.3, Pax4/6, and Arx, all have been implicated in controlling differentiation of mature endocrine cell types in the stomach38, 70-72.
While it is mostly not clear what controls the specification of non-endocrine cell lineages within the stomach, some factors have been implicated in maturation of those cell types. The transcription factor Spdef has been shown to be crucial for antral deep mucous cell maturation73. Foxq1 is necessary for the expression of Muc5ac in pit cells (MUC5AC is the key mucin protein secreted by these cells) 74. Xbp1 and Mist1 (Bhlha15) are important for the ultrastructural maturation of chief cells75, 76. Specifically, they coordinate the architectural changes necessary for these cells to become regulated secretory factories. In their absence, chief cells fail to generate a dense rough endoplasmic reticulum network and do not make large zymogen-containing vesicles. Mucous neck cells, the progenitors for chief cells, emerge in rodents around the time of weaning in a process that depends in part on TGFα and the EGF receptor77, 78, though whether these play a role in maturation or specification is not known.
Adult Homeostasis
Stem Cells
The isthmus of the corpus epithelium contains a continuously proliferating cell population that lacks any differentiated nuclear and cytoplasmic features (e.g., secretory granules or specialized organelles). Nucleotide analog labeling studies (e.g., 3H-thymidine or BrdU) show that label is most frequently incorporated in isthmal cells with those morphologically immature characteristics, and this cell lineage has been termed the “granule-free” stem cell61. Pulse-chase experiments with such analogs show that the labeled nucleotides spread bidirectionally from the isthmus. Karam and Leblond hypothesized that the granule-free stem cell directly gave rise to progenitors that were immature versions of each of the mature corpus lineages. Some of the earliest cells to incorporate label were cells characterized by ultrastructural features of immature pit cells (e.g., scant but distinctive pit cell mucous granules79). Label spread more slowly in the other direction (i.e., towards the base and away from the gastric lumen). The cells that show early incorporation of label in that direction commonly have early/immature mucous neck cell features80. One-two weeks after injection of labeled nucleotides, label appears in the pre-zymogenic chief cells at the top of the base, those with features characteristic of both mucous neck cells and zymogenic cells76, 80. It appears eventually, also in parietal cells and endocrine cells, first in the isthmus area81, 82. In pulse-chase experiments wherein the nucleotides are given only once – as opposed to continuously -- label is typically not retained for longer than a few days in either the immature (presumptive progenitor) cells or in the granule-free cells. Rather, label is retained long-term only in mature parietal, chief, and endocrine cells. The simplest interpretation of these observations is that the undifferentiated, granule-free isthmal cell is a constitutively active multipotent stem cell that can give rise to and replenish all the mature cell lineages (Fig. 3). However, this has not been formally proven.
Other than such studies, wherein lineage relationships are inferred from morphology and labeled nucleotide migration patterns, there is little else known about transitions from the stem cell to progenitors and lineage-committed cells in the corpus. The limited state of understanding in the gastric corpus is in marked contrast to that in the small intestine, where numerous markers of crypt-based cells with stem cell potential have been identified over the past ten years83-87. Strikingly, there is still neither a specific molecular marker nor a specific promoter whose expression is restricted to an undifferentiated isthmal cell in the corpus that has yet been identified.
Several studies using chimeric mice and mosaic silencing of an x-linked transgene in female mice suggest that stomach glands start off polyclonal but become monoclonal over time62, 88-90. These results thus support the single gastric unit stem cell hypothesis. However, Bjerknes and Cheng found patterns of mutant clones that showed that, as mice age, there might be other progenitor-progeny relationships outside the dogma of the single, long-lived, multipotent stem cell proposed by Karam and Leblond62. In adult mice, Bjerknes and Cheng saw units that seemingly had stable labeling restricted to specific single lineages, suggesting that there might also be long-lived, lineage committed progenitor cells rather than the transient ones hypothesized by Karam and Leblond. We have provided a cartoon to distinguish the two models (i.e., a multipotent, undifferentiated stem cell giving rise to all lineages vs. multiple long-lived, lineage-committed progenitors, each fueling only their specific lineage; Fig. 4).
Genetic lineage tracing experiments that have been attempted in the stomach suffer from the caveat that the promoters used to drive lineage-tracing are also expressed (usually much more strongly) in differentiated cells. For example, long-term lineage tracing in adult animals using the Sox2 promoter suggested that some Sox2-promoter-expressing cells have stem cell function with the capacity for self-renewal and differentiation into all lineages in the corpus. Rare, highly Sox2+ cells were suggested to be the most stem-like. Interestingly, those cells localized to the base of corpus units, not the isthmus91. SOX2 protein can be found in many cell lineages throughout the corpus at mid-to-low levels and can even be used as a marker in humans of gastric differentiation relative to intestinal92, 93. Other lineage tracing studies have focused on marking mature chief cells using the Troy or Mist1 promoters94. Long-term lineage tracing using those promoters suggested that chief cells can also act as stem cells and give rise to all the cell lineages within the corpus. MIST1 protein and RNA are almost exclusive to mature chief cells, and TROY is restricted to a handful of chief and parietal cells. These results indicate that differentiated chief cells have the potential to serve as stem cells in some situations, albeit, such functional stem cell activity in chief cells seems relatively rare, at least during homeostasis95. Recent studies have indicated that rare cells labeled with a Mist1CreER knock-in allele can also be found in the isthmus of the corpus96. These occasional, isthmus-localized cells that express the Mist1 promoter could be another source of stemness in the corpus. However, the molecular/cellular identity of those cells is defined only by this spurious Mist1CreER expression, given that neither the endogenous Mist1 transcript, nor the MIST1 protein has been shown to be expressed outside of chief cells in wildtype mice.
Definitive lineage tracing studies in the stomach have also been hampered by a technical problem that doesn’t affect to the same degree other gastrointestinal organs, like small intestine and pancreas, where lineage tracing has been used to great effect. The vast majority of genetic lineage tracing tools use a modified Cre recombinase that requires binding tamoxifen to be transported to the nucleus where it can activate reporter genes or other genetic tools (CreER). Unfortunately, for gastric researchers, tamoxifen induces parietal cell death and chief cell metaplasia when delivered above a threshold dose97-99. Thus, inducible lineage tracing using CreER with tamoxifen can be confounding in the stomach because it may induce non-homeostatic patterns of differentiation with increased cellular plasticity95. The stomach is also particularly sensitive to high doses of Cre itself100. It would be ideal to develop more stomach-lineage-specific promoters and induce lineage tracing with methods like tetracycline-inducible systems or estrogen receptor agonists that don’t induce injury. Furthermore, any lineage tracing in the stomach should be performed with proper controls: mice homozygous for floxed alleles but lacking Cre recombinase expression and mice with Cre recombinase but with a non-floxed allele of the gene of interest.
To highlight how our understanding of stem cell dynamics in the intestine is more advanced than in the corpus, we point out how CreER driven by the Lgr5 promoter is an efficient marker of functional stem cell activity in the intestine. Lgr5CreER is expressed at higher levels in the presumptive stem cells in the small intestine than in differentiated progeny. Importantly, both the endogenous Lgr5 transcript and LGR5 protein are also expressed preferentially in the presumptive stem cell83. Lgr5CreER can be used during homeostasis to trace labeled cells, and all cell lineages can be seen eventually to derive from Lgr5-promoter expressing, crypt-resident (presumptive stem) cells with undifferentiated features. Lgr5 promoter-based studies corroborate other studies that, together, make it seem incontrovertible that there is a population of constitutively active, long-lived multipotent stem cells in the small intestine.
Cellular Differentiation and Maturation in the Corpus Epithelium
Whereas the gastric epithelial stem cell in the adult corpus remains unidentified, there has been some beginning characterization of the patterns of molecular and cellular differentiation of the various mature cell lineages deriving from that stem cell. One strong line of evidence supports an interesting differentiation pattern wherein mucous neck cells give rise to chief cells, a differentiation step that has been termed a “transdifferentiation” to chief cells76, 78, 101. Evidence supporting the lineage relationship between neck and chief cells is circumstantial but varied and relatively abundant. For one, there are situations where neck and chief cell markers are co-expressed. During postnatal maturation, gastric units contain cells with characteristics of both neck and chief cells60. After maturation, units in the corpus harbor similar transitional cells with characteristics of both cell populations by ultrastructural and gene expression analysis76, 80, 101, 102. When the stomach is injured, metaplastic cells arising from the chief cell lineage express both neck and chief cell markers6, 95, 101. Furthermore, lineage tracing studies using the Tff2 promoter have suggested that parietal cells and mucous neck/chief cells are derived from a common progenitor pool103. Finally, slowing maturation of chief cells by deleting either Mist1 or Xbp1 leads to increased cells with neck-chief transitional characteristics75, 76, 101.
The surface mucous (pit/foveolar) cells clearly arise rapidly from a progenitor in the isthmus. The nature of the progenitor has not been established but must be either a committed pit-specific long-lived progenitor or the canonical multipotent stem cell (or both). Interestingly, we have observed that decreased proliferation in the isthmal progenitor zone tends to have effects on pit cells more than the deeper glandular cells104, indicating that much of the proliferation in the isthmus, at least under normal conditions, is directed toward surface mucous cell replenishment. As mentioned earlier the transcription factor FOXQ1 is involved in pit cell differentiation, as it is required for expression of the key component of the pit cell mucous granules (MUC5AC), though it is not required for specification of the lineage itself74.
A number of signaling pathways have been shown to be active during stomach homeostasis and affect cell behavior. Notch signaling is active in the isthmus of the corpus and promotes proliferation within this region105. Ectopic Notch signaling driven by a parietal cell-lineage-specific promoter blocked differentiation and maintained progenitor characteristics in differentiating parietal cells105. Inhibition of the BMP signaling pathway promotes increased cell proliferation in the adult stomach: glands contained fewer parietal cells and more transitional cells (cells with both neck and zymogenic chief cell characteristics – similar to the metaplastic or transitional cells mentioned above) at the base of the unit106, indicating that BMP signals regulate progenitor proliferation and cell maturation in the corpus.
Gastrin is a hormone produced by G-cells in the antrum. The primary physiological role of gastrin is to promote acid secretion by activating ECL and parietal cells. Absence of gastrin causes decreased cellular proliferation in the corpus and leads to generation of immature parietal and ECL cells,107-110 while overexpression of gastrin causes increased proliferation of those cell populations111. Parietal cell production of Shh is an important regulator of gastrin production. In the absence of this source of Shh, excess gastrin is produced by G-cells, and pit cells in the corpus have increased proliferation112.
Antral homeostasis
The antrum is considerably less complex then the corpus (in organization and number of cell types). The cell lineages also turn over faster. Continuously proliferating cells in the antrum are located at the isthmus of the unit. In the isthmus, the Leblond group identified the most actively proliferating cells as also being the least differentiated ultrastructurally (like the “granule-free” presumptive stem cell in the isthmus of the corpus). In the antrum, the isthmus is much nearer the base than in the corpus, in a pattern more resembling the large intestine. In pulse-chase experiments, labeled DNA spreads both upward to the lumen and further down into the base from this isthmus zone113 (Fig. 1).
In contrast to the corpus, markers and gene promoters have been shown to efficiently label cells with multipotent progenitor capacity. For example, as in the small intestine, Lgr5 shows a pattern of homeostatic expression that is confined to a specific cell population that frequently and efficiently can be traced into progeny that include all the cell lineages in the antrum and cardia, but not the corpus114. Similarly, Cck2r based-lineage tracing labeled a “+4” (the designation of “+4” is borrowed from the intestine, wherein cells have traditionally been numbered from the most basal cell upward to the lumen) antral cell that also has stem cell potential and was shown to give rise to Lgr5+ antral cells115. Additionally, Villin- and Sox2-promoter-based lineage tracing also label rare cells in antral glands that exhibit functional stem cell characteristics91, 116. It is not yet clear what the relationship among all these cell populations with stem cell capacity is yet. The LGR5+ cells are clearly not the granule-free, isthmal antral cells113, as they are commonly located at the very base of the antral unit, not the isthmus, and they show ultrastructural features of differentiation113, 114. The CCK2R and LGR5 populations seem to be overlapping, at least functionally, but are distinct from each other. Cells labeled by Villin are rare and activated only by inflammation116. Sox2, on the other hand, is expressed in many cells, so it is likely not specific to a defined stem cell91. It is possible that cells in the antrum are plastic, so that many cells can serve as stem cells even homeostatically. Antral glands also undergo relatively frequent fission events, where one gland gives rise to another62, 89, 117, so perhaps some of the markers label cells that are not constitutive stem cells but that drive budding off of new glands. In sum, Sox2, Lgr5, and Villin are not principally expressed in the zone where the least differentiated, most proliferative cells are. That is in contrast to the intestine where the Lgr5-expressing (crypt-base-columnar) cells are the most proliferative and the least ultrastructurally differentiated cells. Perhaps, thus, a marker of isthmal, antral stem cells that is equivalent to LGR5 in the intestine, has yet to be identified in the stomach.
In the antrum, Notch signaling regulates the behavior of Lgr5+ antral stem cells. Inhibition of Notch signaling promotes mucous and endocrine cell differentiation whereas activating the pathway stops differentiation118. BMP signaling, through BMPR1A, regulates the proliferation and differentiation of mucous cells in the antrum100, 119. In the absence of BMPR1A, antral mucous cells hyperproliferate and fail to express the mucin MUC5AC119. Mutations in the BMP family are known to cause juvenile polyposis syndrome which presents with polyps throughout the gastrointestinal tract, including the antrum120. Finally, given that LGR5 is expressed and may regulate stem cell activity in the antrum, there may be a role for Wnt signaling in regulating antral homeostasis, as LGR5 is a co-receptor for canonical Wnt signals121.
Conclusions
Many facets of stomach specification remain understudied or unexplored. While it is clear that tissue interactions between the gastric endoderm and mesoderm are important for gastric development, there is still scant knowledge about how naïve endodermal progenitors become specified to the gastric progenitor state. There is equally poor understanding about the factors that control the specification of gastric lineages during development and adult homeostasis, other than some initial inroads into outlining the origins of endocrine lineages. Many more studies are needed to determine which cell types have stem cell properties in the adult stomach (in particular the corpus) and their relative contribution during homeostasis and disease/injury conditions. Such studies will depend on development of promoter-based tools that, like the intestine, are specific for stem cells and not expressed in differentiated cells. Preferably, such tools would not depend on the possibly confounding agent tamoxifen. Other new potential tools to help sort out gastric specification are being developed. Recent reports have described the derivation of mouse and human gastric organoids derived either from adult stomach94, 122-126 or via differentiation from induced pluripotent stem cells40. Potentially through the manipulation of these cells, the field might have a new approach to better understand the pathways and factors controlling stemness and specification of gastric lineages. The generation of such new tools to study these processes is an important first step to exploring the mechanisms that control gastric specification.
Synopsis.
This review details the current understanding of gastric epithelial tissue and cell lineage specification in development and adult homeostasis
Acknowledgments
(none)
Funding information: NIH NIDDK awards DK094989, DK052574, DK052574, American Cancer Society award RSG-12-171-01-LIB, Washington University Alvin J. Siteman Cancer Center (supported by NCI P30 CA91842) pre-SPORE award (JCM); NCI CA009547 (SGW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflicts of interest exist.
Author contributions: SGW and JCM wrote the manuscript and prepared the figures
References
- 1.Hunt RH, Camilleri M, Crowe SE, et al. The stomach in health and disease. Gut. 2015;64:1650–68. doi: 10.1136/gutjnl-2014-307595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart BW, Wild C, International Agency for Research on Cancer et al. World cancer report 2014. International Agency for Research on Cancer WHO Press; Lyon, France Geneva, Switzerland: 2014. [Google Scholar]
- 3.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–16. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Lennerz JK, Kim SH, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–33. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PubMed] [Google Scholar]
- 6.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi E, Roland JT, Barlow BJ, et al. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–20. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JS, Nakane PK, Allen RH. Immunocytochemical localization of human intrinsic factor: the nonstimulated stomach. Gastroenterology. 1980;79:493–502. [PubMed] [Google Scholar]
- 9.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–51. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–91. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Khurana S, Mills JC. The gastric mucosa development and differentiation. Prog Mol Biol Transl Sci. 2010;96:93–115. doi: 10.1016/B978-0-12-381280-3.00004-X. [DOI] [PubMed] [Google Scholar]
- 12.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–20. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 13.Bayha E, Jorgensen MC, Serup P, et al. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Dolle P, Cardoso WV, et al. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol. 2006;297:433–45. doi: 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–72. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 16.Dessimoz J, Opoka R, Kordich JJ, et al. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood RI, Maehr R, Mazzoni EO, et al. Wnt signaling specifies and patterns intestinal endoderm. Mechanisms of Development. 2011;128:387–400. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiso N, Filippi A, Pauls S, et al. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 19.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–13. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–52. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 21.Bort R, Martinez-Barbera JP, Beddington RS, et al. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- 22.Mao J, Kim BM, Rajurkar M, et al. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–9. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–78. doi: 10.1016/j.mod.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee CS, Friedman JR, Fulmer JT, et al. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–7. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 25.Gao N, LeLay J, Vatamaniuk MZ, et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–48. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye DZ, Kaestner KH. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology. 2009;137:2052–62. doi: 10.1053/j.gastro.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 28.Kuo CT, Morrisey EE, Anandappa R, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–60. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin JD, Lin Q, Duncan SA, et al. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–72. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 30.Koutsourakis M, Langeveld A, Patient R, et al. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–32. [PubMed] [Google Scholar]
- 31.Jacobsen CM, Narita N, Bielinska M, et al. Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev Biol. 2002;241:34–46. doi: 10.1006/dbio.2001.0424. [DOI] [PubMed] [Google Scholar]
- 32.Que J, Luo X, Schwartz RJ, et al. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que J, Okubo T, Goldenring JR, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–31. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghoebir L, Bakker ER, Mills JC, et al. SOX2 redirects the developmental fate of the intestinal epithelium toward a premature gastric phenotype. J Mol Cell Biol. 2012;4:377–85. doi: 10.1093/jmcb/mjs030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–99. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Qin R, Liu B, et al. Multilayered epithelium in a rat model and human Barrett's esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol. 2008;8:1. doi: 10.1186/1471-230X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura S, Settle SH, Leys CM, et al. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier's disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Larsson LI, Madsen OD, Serup P, et al. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–84. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- 39.Haumaitre C, Barbacci E, Jenny M, et al. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102:1490–5. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCracken KW, Cata EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–4. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CS, Sund NJ, Behr R, et al. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–95. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Choi E, Kraus MR, Lemaire LA, et al. Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem Cells. 2012;30:2297–308. doi: 10.1002/stem.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harfe BD, Scherz PJ, Nissim S, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–28. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi K, Yasugi S, Mizuno T. Pepsinogen gene transcription induced in heterologous epithelial-mesenchymal recombinations of chicken endoderms and glandular stomach mesenchyme. Development. 1988;103:725–31. doi: 10.1242/dev.103.4.725. [DOI] [PubMed] [Google Scholar]
- 45.Urase K, Fukuda K, Ishii Y, et al. Analysis of mesenchymal influence on the pepsinogen gene expression in the epithelium of chicken embryonic digestive tract. Rouxs Archives of Developmental Biology. 1996;205:382–390. doi: 10.1007/BF00377218. [DOI] [PubMed] [Google Scholar]
- 46.Takiguchi K, Yasugi S, Mizuno T. Gizzard Epithelium of Chick-Embryos Can Express Embryonic Pepsinogen Antigen, a Marker Protein of Proventriculus. Rouxs Archives of Developmental Biology. 1986;195:475–483. doi: 10.1007/BF00375887. [DOI] [PubMed] [Google Scholar]
- 47.Yasugi S, Matsushita S, Mizuno T. Gland Formation Induced in the Allantoic and Small Intestinal Endoderm by the Proventricular Mesenchyme Is Not Coupled with Pepsinogen Expression. Differentiation. 1985;30:47–52. [Google Scholar]
- 48.Narita T, Saitoh K, Kameda T, et al. BMPs are necessary for stomach gland formation in the chicken embryo: a study using virally induced BMP-2 and Noggin expression. Development. 2000;127:981–8. doi: 10.1242/dev.127.5.981. [DOI] [PubMed] [Google Scholar]
- 49.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 50.El-Zaatari M, Saqui-Salces M, Waghray M, et al. Sonic hedgehog in gastric physiology and neoplastic transformation: friend or foe? Curr Opin Endocrinol Diabetes Obes. 2009;16:60–5. doi: 10.1097/MED.0b013e328320a821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer-Dene B, Sala FG, Bellusci S, et al. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology. 2006;130:1233–44. doi: 10.1053/j.gastro.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Nyeng P, Norgaard GA, Kobberup S, et al. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. doi: 10.1016/j.ydbio.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speer AL, Al Alam D, Sala FG, et al. Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis. PLoS One. 2012;7:e49127. doi: 10.1371/journal.pone.0049127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim BM, Buchner G, Miletich I, et al. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–22. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Kim BM, Miletich I, Mao J, et al. Independent functions and mechanisms for homeobox gene Barx1 in patterning mouse stomach and spleen. Development. 2007;134:3603–13. doi: 10.1242/dev.009308. [DOI] [PubMed] [Google Scholar]
- 56.Verzi MP, Stanfel MN, Moses KA, et al. Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology. 2009;136:1701–10. doi: 10.1053/j.gastro.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Self M, Geng X, Oliver G. Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev Biol. 2009;334:409–17. doi: 10.1016/j.ydbio.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Udager AM, Prakash A, Saenz DA, et al. Proper development of the outer longitudinal smooth muscle of the mouse pylorus requires Nkx2-5 and Gata3. Gastroenterology. 2014;146:157–65. doi: 10.1053/j.gastro.2013.10.008. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prakash A, Udager AM, Saenz DA, et al. Roles for Nkx2-5 and Gata3 in the ontogeny of the murine smooth muscle gastric ligaments. Am J Physiol Gastrointest Liver Physiol. 2014;307:G430–6. doi: 10.1152/ajpgi.00360.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin- interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–51. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–79. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 62.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–77. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 63.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–10. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Kokubu H, Ohtsuka T, Kageyama R. Mash1 is required for neuroendocrine cell development in the glandular stomach. Genes Cells. 2008;13:41–51. doi: 10.1111/j.1365-2443.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 65.Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–47. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee CS, Perreault N, Brestelli JE, et al. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–97. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li HJ, Johnston B, Aiello D, et al. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology. 2014;146:754–764. doi: 10.1053/j.gastro.2013.11.048. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mastracci TL, Sussel L. The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdiscip Rev Dev Biol. 2012;1:609–28. doi: 10.1002/wdev.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li HJ, Ray SK, Singh NK, et al. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsson LI, St-Onge L, Hougaard DM, et al. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–9. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- 71.Choi MY, Romer AI, Wang Y, et al. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol Cell Biol. 2008;28:3208–18. doi: 10.1128/MCB.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du A, McCracken KW, Walp ER, et al. Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol. 2012;365:175–88. doi: 10.1016/j.ydbio.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horst D, Gu X, Bhasin M, et al. Requirement of the epithelium-specific Ets transcription factor Spdef for mucous gland cell function in the gastric antrum. J Biol Chem. 2010;285:35047–55. doi: 10.1074/jbc.M110.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verzi MP, Khan AH, Ito S, et al. Transcription factor foxq1 controls mucin gene expression and granule content in mouse stomach surface mucous cells. Gastroenterology. 2008;135:591–600. doi: 10.1053/j.gastro.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huh WJ, Esen E, Geahlen JH, et al. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139:2038–49. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey VG, Doherty JM, Chen CC, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 77.Osaki LH, Curi MA, Alvares EP, et al. Early weaning accelerates the differentiation of mucous neck cells in rat gastric mucosa: possible role of TGFalpha/EGFR. Differentiation. 2010;79:48–56. doi: 10.1016/j.diff.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Hanby AM, Poulsom R, Playford RJ, et al. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–7. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 79.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–96. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 80.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 81.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–32. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 82.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–40. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 83.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 84.Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Powell AE, Wang Y, Li Y, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–58. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeda N, Jain R, LeBoeuf MR, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–4. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tatematsu M, Fukami H, Yamamoto M, et al. Clonal analysis of glandular stomach carcinogenesis in C3H/HeN<==>BALB/c chimeric mice treated with N-methyl-N-nitrosourea. Cancer Lett. 1994;83:37–42. doi: 10.1016/0304-3835(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 89.Nomura S, Esumi H, Job C, et al. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–35. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 90.Thompson M, Fleming KA, Evans DJ, et al. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477–81. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- 91.Arnold K, Sarkar A, Yram MA, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khalili M, Vasei M, Khalili D, et al. Downregulation of the Genes Involved in Reprogramming (SOX2, c-MYC, miR- 302, miR-145, and P21) in Gastric Adenocarcinoma. J Gastrointest Cancer. 2015;46:251–8. doi: 10.1007/s12029-015-9695-2. [DOI] [PubMed] [Google Scholar]
- 93.van Olphen S, Biermann K, Spaander MC, et al. SOX2 as a Novel Marker to Predict Neoplastic Progression in Barrett's Esophagus. Am J Gastroenterol. 2015;110:1420–8. doi: 10.1038/ajg.2015.260. [DOI] [PubMed] [Google Scholar]
- 94.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–68. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800–14. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saenz JB, Burclaff J, Mills JC. Gastrointestinal Physiology and Diseases: Methods and Protocols. Springer; 2015. Modeling murine gastric metaplasia through tamoxifen-induced acute parietal cell loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maeda Y, Echizen K, Oshima H, et al. Myeloid Differentiation Factor 88 Signaling in Bone Marrow-Derived Cells Promotes Gastric Tumorigenesis by Generation of Inflammatory Microenvironment. Cancer Prev Res (Phila) 2016;9:253–63. doi: 10.1158/1940-6207.CAPR-15-0315. [DOI] [PubMed] [Google Scholar]
- 100.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of "floxed" alleles. Am J Physiol Gastrointest Liver Physiol. 2010;299:G368–80. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bredemeyer AJ, Geahlen JH, Weis VG, et al. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–24. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzuki S, Tsuyama S, Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res. 1983;233:475–84. doi: 10.1007/BF00212218. [DOI] [PubMed] [Google Scholar]
- 103.Quante M, Marrache F, Goldenring JR, et al. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027. doi: 10.1053/j.gastro.2010.08.003. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khurana SS, Riehl TE, Moore BD, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–97. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–88. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinohara M, Mao M, Keeley TM, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friis-Hansen L, Sundler F, Li Y, et al. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–8. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 108.Koh TJ, Goldenring JR, Ito S, et al. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–25. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 109.Ohning GV, Wong HC, Lloyd KC, et al. Gastrin mediates the gastric mucosal proliferative response to feeding. Am J Physiol. 1996;271:G470–6. doi: 10.1152/ajpgi.1996.271.3.G470. [DOI] [PubMed] [Google Scholar]
- 110.Hinkle KL, Bane GC, Jazayeri A, et al. Enhanced calcium signaling and acid secretion in parietal cells isolated from gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G145–53. doi: 10.1152/ajpgi.00283.2002. [DOI] [PubMed] [Google Scholar]
- 111.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao C, Ogle SA, Schumacher MA, et al. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–61. doi: 10.1053/j.gastro.2009.11.002. 561 e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–24. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 114.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 115.Hayakawa Y, Jin G, Wang H, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–53. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiao XT, Ziel JW, McKimpson W, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–98. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee ER. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am J Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- 118.Demitrack ES, Gifford GB, Keeley TM, et al. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–36. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bleuming SA, He XC, Kodach LL, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–55. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 120.Brosens LA, Langeveld D, van Hattem WA, et al. Juvenile polyposis syndrome. World J Gastroenterol. 2011;17:4839–44. doi: 10.3748/wjg.v17.i44.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–7. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 122.Gifford GB, Demitrack ES, Keeley TM, et al. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2016 doi: 10.1136/gutjnl-2015-310811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schumacher MA, Aihara E, Feng R, et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–27. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li X, Nadauld L, Ootani A, et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–77. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore BD, Jin RU, Osaki L, et al. Identification of alanyl aminopeptidase (CD13) as a surface marker for isolation of mature gastric zymogenic chief cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G955–64. doi: 10.1152/ajpgi.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schlaermann P, Toelle B, Berger H, et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–13. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]