Figure 5. Mutation at single or multiple sites on clathrin NTD disrupts binding to peptide motifs.
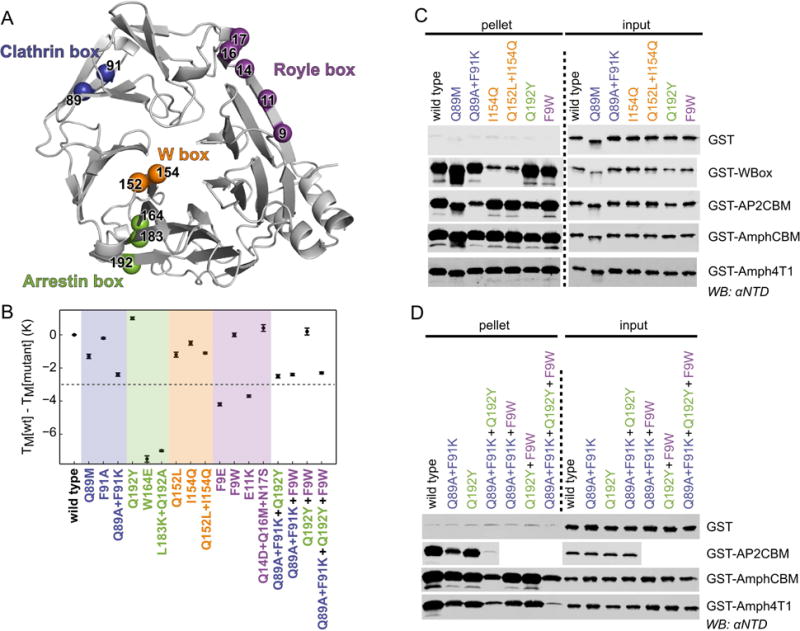
(A) Ribbon representation of NTD (grey) showing the location of residues that were mutated on their own or in combination to disrupt peptide binding at the clathrin box (blue), arrestin box (green), W box (orange) and Royle box (purple). (B) Thermal stability of single- or multiple-site mutations of NTD as determined by differential scanning fluorimetry. The melting temperatures (TM) of mutants relative to that of wild-type His-NTD are shown (error bars represent the standard deviation of measurement in triplicate). Mutations that perturb the TM by more than 3 K (dotted line) were not considered further. (C, D) Capture of NTD mutants by GST-tagged clathrin-binding peptides. Purified recombinant wild-type or mutant His-NTD-NEMO was incubated with glutathione sepharose pre-loaded with GST-tagged “bait” proteins. After washing, proteins bound to the beads (pellet) were subjected to SDS-PAGE and immunoblotting (WB) using an antibody that recognizes clathrin NTD (αNTD).
