Abstract
Background and Purpose
One class of post-stroke restorative therapy focuses on promoting axon outgrowth by blocking myelin-based inhibitory proteins such as myelin-associated glycoprotein (MAG). The purpose of the current study was to extend preclinical and clinical findings of GSK249320, a humanized monoclonal antibody to MAG with disabled Fc region, to explore effects on motor outcomes post-stroke.
Methods
In this phase IIb double-blind, randomized, placebo-controlled study, patients at 30 centers with ischemic stroke 24–72 hours prior and gait deficits were randomized to two IV infusions of GSK249320 or placebo. Primary outcome measure was change in gait velocity from baseline to Day 90.
Results
A total of 134 subjects were randomized between May 2013–July 2014. The two groups were overall well matched at baseline. The study was stopped at the pre-specified interim analysis because the treatment difference met the predefined futility criteria cutoff; change in gait velocity to Day 90 was 0.55±0.46 (mean±SD) in the GSK249320 group and 0.56±0.50 for placebo. Secondary endpoints including upper extremity function were concordant. The two IV infusions of GSK249320 were well tolerated. No neutralizing antibodies to GSK249320 were detected.
Conclusions
GSK249320 within 72 hours of stroke demonstrated no improvement on gait velocity vs. placebo. Possible reasons include challenges translating findings into humans and no direct evidence that the therapy reached the biological target. The antibody was well tolerated and showed low immunogenicity, findings potentially useful to future studies aiming to use a monoclonal antibody to modify activity in specific biological pathways to improve recovery from stroke.
Clinical Trial Registration Information
Keywords: stroke recovery, axon, gait velocity, clinical trial
Subject terms: clinical studies, ischemic stroke, rehabilitation
After the injury from an acute stroke, numerous restorative events evolve within the brain. Targeting these events therapeutically may augment post-stroke neural repair and favorably impact long-term outcome1. Numerous biological targets are under study to develop restorative therapies. One class of therapy focuses on promoting recovery after stroke by blocking myelin-based inhibitory proteins that inhibit axon outgrowth. Three major inhibitors of such growth have been identified, one being myelin-associated glycoprotein (MAG). After stroke, MAG levels spontaneously increase in penumbra2, suggesting MAG may be a useful target to promote neural repair, an idea bolstered by prior observations that MAG blockade promotes axonal growth3–5.
The main objective of the current study was to determine whether a monoclonal antibody targeting MAG improves stroke recovery in patients with ischemic stroke. The specific therapy under study was GSK249320, an IgG1-type humanized monoclonal antibody to MAG with disabled Fc region. Anti-MAG antibodies have been shown to neutralize MAG-mediated inhibition in pre-clinical studies6 and to promote regeneration after peripheral nerve injury7, 8. Blocking the action of a related protein, Nogo, seven days after ischemic stroke in rats improved behavioral recovery by promoting axonal growth9. The preclinical program for GSK249320 included rodent studies that found that the antibody penetrated the infarct site and had small but significant effects on behavioral outcomes when initiated 24 hours post-stroke without affecting infarct volume10, and primate studies in which intravenous (IV) infusion of GSK249320 beginning 24 hours after experimental ischemic infarct facilitated behavioral recovery11. GSK249320 was found to be safe in healthy human subjects12, and a recent randomized placebo-controlled Phase II trial in patients 24–72 hours after ischemic stroke also found the antibody to be safe, and suggested potential efficacy for improving recovery of gait13.
The current study built on these findings as a Phase IIb double-blind, randomized, placebo controlled, multicenter study. Patients with ischemic stroke 24–72 hours prior and deficits in gait were randomized to receive two IV infusions of GSK249320 or placebo. The primary outcome measure was change from baseline to Day 90 in gait velocity, which is valid, reliable, and sensitive after stroke14, 15. The study was stopped at the interim analysis because there was insufficient evidence to justify continuing the study given that the observed difference between treatment groups met the pre-defined futility cutoff.
Methods
Study overview
Thirty centers across 4 countries enrolled subjects in the study, between May 2013–July 2014. The study was approved by each site’s Institutional Review Board. All subjects, or surrogates, gave written informed consent. This study was registered (clinicaltrials.gov NCT01808261). Participation spanned six visits from baseline-Day 180. Key entry/exclusion criteria appear in Table 1. See also Supplemental Material.
Table 1.
Key entry and exclusion criteria
| Entry criteria |
| Radiologic confirmed supratentorial ischemic stroke; non-lacunar (either >15mm diameter in one direction or >4cc volume) |
| Stroke onset within 24–72 hours of IP infusion |
| NIHSS score 3–21 |
| Leg motor deficit: NIHSS Q6 score 1–4 |
| Impaired walking ability: gait velocity≤0.8m/s |
| Aged 18–90 years |
| Expectation subject will receive standard physical, occupational and speech rehabilitation therapy as indicated for post-stroke deficits. |
| Exclusion criteria |
| Ability to walk>0.8m/s per Gait Velocity assessment. |
| Symptomatic stroke <3 months before study entry |
| Significant pre-stroke disability: Rankin score>2 before index stroke |
| Poorly responsive: NIHSS Q1a score 2 or 3 |
| Significant aphasia |
| Pre-existing significant gait deficit, chronic liver disease, or prolonged QTc interval |
| Pre-existing active poorly controlled neurological or psychiatric disease |
| Expected death due to index stroke or other pre-existing condition |
| Participation in another investigational study targeting stroke recovery during study |
| MRI contraindication |
| Pregnant/lactating |
Randomization
Subjects were centrally randomized to GSK249320 15mg/kg or placebo in a 1:1 allocation ratio, using permuted blocks, with treatment stratified according to baseline gait velocity (0m/s, > 0m/s to < 0.4m/s, or 0.4m/s to 0.8m/s). See also Supplemental Material.
Study assessments
At baseline, prior to first infusion and thus <72 hours post-stroke, assessments included NIHSS, modified Rankin Scale, gait velocity, and Box & Blocks (# blocks transferred during 1 minute). All study assessors were formally trained and certified in each of these outcome measures (see Supplemental Material). Patients and assessors were blinded at all times. These were serially evaluated over the remaining five visits, as was the amount of rehabilitation (physical and occupational) therapy that patients received. Safety assessments included vital signs, clinical labs, ECGs, suicidality, adverse events (AE), serious adverse events (SAE), and falls, and were monitored by the internal Safety Review Committee (iSRC). Blood samples were collected at baseline, pre- and post-dosing of IP at Visit 2 (Day 6), as well as at Visits 3 and 6 (Day 30 and 180, respectively), or at the time of study withdrawal if applicable, from which free serum MAG levels and GSK249320 levels were measured. See also Supplemental Material.
Data analysis
The primary efficacy endpoint was the mean change in gait velocity from baseline to Day 90. To test the hypothesis that treatment with GSK249320 leads to an improvement of change in gait velocity compared to placebo at Day 90, a repeated measures mixed effects model was used in a Bayesian framework, including fixed effects for treatment, visit, age, sex, treatment by visit interaction, baseline mean gait velocity by visit interaction, and baseline NIHSS by visit interaction. For additional information, see Supplemental Material. At the end of study, a positive signal of efficacy was to be declared if the posterior probability that the true improvement over placebo (GSK249320-placebo) was greater than zero is >95%, and a negative signal of efficacy was to be declared if the posterior probability that the true improvement over placebo is greater than zero is <85%; otherwise the result was to be interpreted as indeterminate. If the true mean gait velocity improvement with GSK249320 is 0.25 m/s over placebo, assuming variance as in the earlier placebo-controlled phase II study of GSK24932013, enrolling 136 subjects with Day 90 data would provide an 85% chance of observing a positive signal of efficacy. Assuming a 16% dropout rate to Day 90, enrollment of 162 subjects was planned. Note that a change in gait velocity of 0.1m/s has been suggested as clinically meaningful in populations with impaired walking speed16 and an increase of 0.16m/s is linked to a meaningful improvement in disability17.
One interim and one headline data analysis were planned during the study. The interim analysis was planned for when approximately 70 subjects completed the Day 90 visit. At that time, the iSRC was to determine if the estimated treatment effect of GSK249320 was likely to be futile based on a pre-specified clinically meaningful treatment effect, i.e., if the posterior probability that the true improvement over placebo is greater than zero is <70%. If the data hit the futility threshold, the iSRC would recommend discontinuation of the study.
The Safety population was defined as subjects who received at least one infusion of IP. The Intent-to-Treat (ITT) population was defined as subjects in the Safety population who underwent at least one post-baseline efficacy assessment, with subjects analyzed according to the treatment to which they were randomized. ITT was the population used for the primary efficacy analysis. The Per Protocol (PP) population was defined as all subjects in the ITT population who were not protocol violators with regards to inclusion/exclusion criteria, unblinding, IP administration, or gait velocity assessments. Subjects who did not receive both infusions of IP were also excluded from the PP population.
Results
Study conduct
A total of 134 subjects were randomized across all four participating countries, including 64 who were enrolled during the 3 months it took for the 70th subject to reach day 90, the futility criteria interim analysis to be completed, and the iSRC to make and communicate the decision to stop the study. Of the 133 that received investigational product, 64 (48%) subjects completed the study and 69 (52%) withdrew from the study or were lost to follow-up (Figure 1). The primary reason for withdrawal was that the study was terminated at the interim analysis. A total of 100 (75%) subjects were in the study for more than 90 days. A total of 116 (87%) subjects received both infusions of IP; one subject received no IP infusions, 10 subjects received only one IP infusion, two subjects received an incorrect dose for one infusion due to incorrect preparation of the dose, and three subjects received less than the full 100mL volume of IP for at least one infusion. Overall, protocol deviations were reported for 109 (81%) subjects, most of which were minor and did not require exclusion from the PP population (Supplementary Table I); all protocol deviations were collected, for transparency, regardless as to whether or not they had an impact on outcome. Of the 134 subjects randomized into the study, 133 were included in the Safety population (Placebo, N=68; GSK249320, N=65), 120 were included in the ITT population (Placebo, N=60; GSK249320, N=60), and 104 were included in the PP population.
Figure 1.
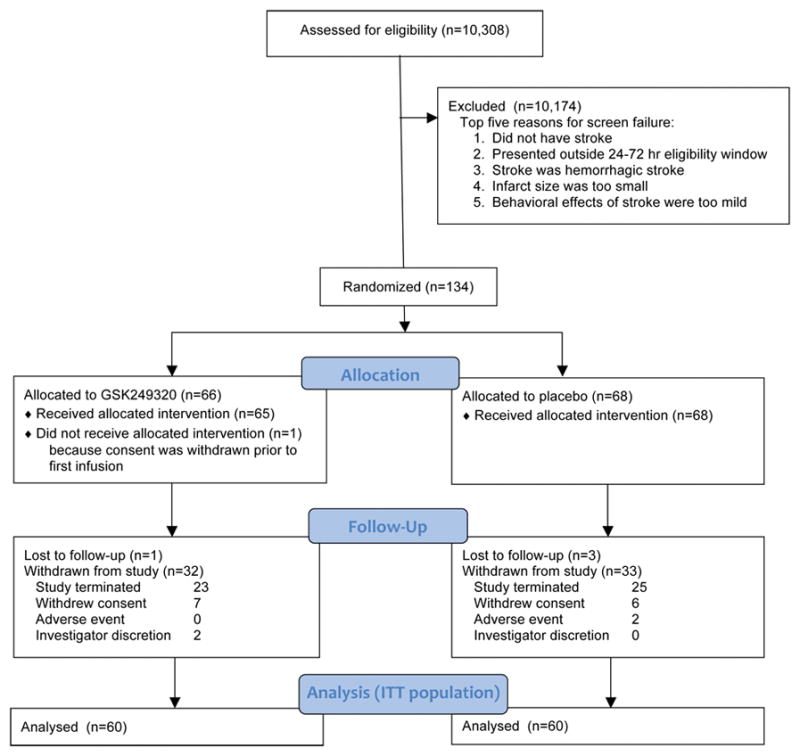
CONSORT diagram.
Subjects
Baseline data (Table 2) were generally balanced across treatment groups. The majority of enrollees (91%) had stroke involving the middle cerebral artery territory. During study participation, the amount of rehabilitation therapy, in minutes, provided to enrollees was substantial and variable, with subjects randomized to GSK249320 receiving a greater amount of therapy (Table 3).
Table 2.
Baseline Clinical Measures and Demographics
| Placebo (N=68) | GSK249320 (N=65) | |
|---|---|---|
|
| ||
| Age (years)* | 67.1±11.2 | 68.2±11.9 |
|
| ||
| Sex (F/M) | 29/39 | 31/34 |
|
| ||
| Hypertension | 51 | 47 |
|
| ||
| Diabetes Mellitus | 22 | 18 |
|
| ||
| Hyperlipidemia | 36 | 28 |
|
| ||
| Atrial fibrillation | 18 | 17 |
|
| ||
| History of Angina Pectoris/MI | 1 | 1 |
|
| ||
| History of Stroke | 0 | 0 |
|
| ||
| Ethnicity | ||
| Hispanic/Latino | 0 | 1 |
| Not Hispanic/Latino | 68 | 64 |
|
| ||
| Race | ||
| White | 62 | 62 |
| African-American/African Heritage | 4 | 2 |
| American Indian/Alaskan Native | 1 | 0 |
| Asian | 1 | 1 |
|
| ||
| Received IV tPA | 29 | 25 |
|
| ||
| Received IA Reperfusion Therapy | 3 | 9 |
|
| ||
| Stroke Subtype | ||
| Large-artery atherosclerosis | 24 | 20 |
| Cardioembolism | 19 | 25 |
| Small-vessel occlusion | 10 | 9 |
| Ischemic stroke other determined etiology | 2 | 2 |
| Ischemic stroke undetermined etiology | 13 | 9 |
|
| ||
| Gait Impairment Stratification | ||
| 0 | 55 | 53 |
| >0–<0.4 | 5 | 5 |
| 0.4–0.8 | 8 | 6 |
| >0.8 | 0 | 1 |
|
| ||
| NIHSS Total Score at Day 1, median (range) | 9.5 (3–20) | 10.0 (3–19) |
|
| ||
| NIHSS Q6 Leg Deficit, Day 1* | 2.4±1.20 | 2.1±1.09 |
|
| ||
| NIHSS Q5 Arm Deficit, Day 1* | 2.7±1.29 | 2.4±1.34 |
|
| ||
| Box & Blocks Score, Day 1* | ||
| Stroke-affected arm | 3.2±7.7 | 4.2±8.4 |
| Non-stroke arm | 25.1±13.3 | 23.3±12.4 |
|
| ||
| Hours, Stroke Onset-First IP Infusion* | 52.7±14.4 | 52.4±13.3 |
Values are for Safety population, except Box & Blocks Score Day 1=Per Protocol population.
mean±SD
Table 3.
Therapy Provided to Enrollees for the Duration of Study Participation
| Placebo (N=52) | GSK249320 (N=52) | |
|---|---|---|
| Physical Therapy | 1,422 [0–10,003] | 1,610 [92–11,285] |
| Occupational Therapy | 771 [0–10,003] | 1,312 [0–11,415] |
| Total Therapy | 2,241 [0–20,006] | 3,264 [184–22,700] |
Results are PP population, median (range), and are minutes of therapy
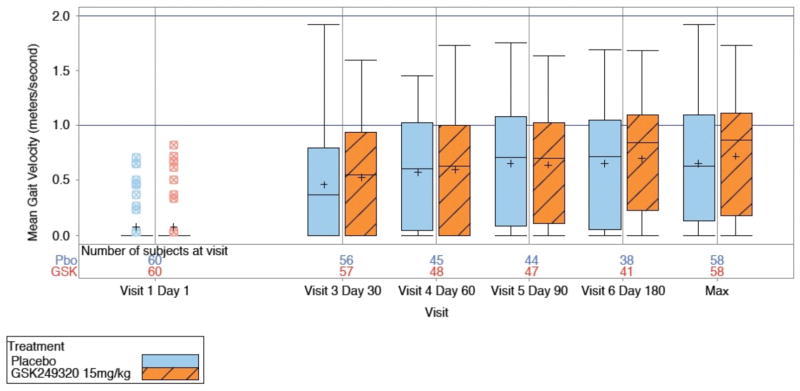
Analysis of treatment efficacy (Figure 2)
Figure 2.
Box-and-whisker plots of gait velocity change over time and maximum value for the two treatment arms (ITT group).
The study was stopped at the interim analysis because the posterior mean treatment difference was 0.027 at Day 90 (95% Credible Interval −0.146, 0.199) and the posterior probability that true treatment difference was greater than 0 was 0.621, which was lower than the predefined futility cutoff of 0.70. Analysis (1) of the PP population, and (2) using the final database including subject data for those subjects with an early withdrawal visit due to study termination, were concordant (Supplemental Material).
Gait velocity data described the proportion of subjects in each gait impairment category (0, >0 to < 0.4m/s, 0.4m/s to 0.8m/s, and >0.8m/s) over time. Most subjects were non-ambulatory at baseline and progressed to some level of ambulation by Day 180, but a review of summary statistics for the secondary endpoints (change in gait impairment category, change in Box & Blocks score, distribution of modified Rankin Scale scores, total NIHSS score) suggests no obvious differences between treatment groups (Table 4).
Table 4.
Study outcomes
| Placebo | GSK249320 | |
|---|---|---|
|
| ||
| Change in gait velocity, baseline-Day 90, mean±SD (ITT) |
n=44 0.56±0.50 |
n=47 0.55±0.46 |
|
| ||
| Change in gait velocity, baseline-Day 180, mean±SD (ITT) |
n=38 0.56±0.48 |
n=41 0.60±0.44 |
|
| ||
| Change in Box & Blocks score, baseline-Day 90, mean±SD (PP) | n=41 | n=40 |
| Stroke-affected arm | 17.1±19.1 | 14.9±16.5 |
| Non-stroke arm | 18.6±15.2 | 14.6±16.4 |
|
| ||
| Subjects falling to Day 90 (Safety) | 15 | 12 |
|
| ||
| modified Rankin Scale score, Day 90(PP) | n=46 | n=45 |
| 0 | 0 | 2 |
| 1 | 7 | 6 |
| 2 | 13 | 11 |
| 3 | 10 | 11 |
| 4 | 14 | 14 |
| 5 | 2 | 1 |
|
| ||
| NIH Stroke Scale score, Day 90, median (IQR) (PP) | 4 (1.25, 8.75) | 4 (1,7) |
Values provided for the population indicated. Gait velocity is in m/sec. ITT was used for the primary efficacy analysis of the primary endpoint (gait velocity), PP was used for secondary endpoints, and the Safety population was used for data on falls.
Analysis of safety
The two IV infusions of GSK249320 were well tolerated as evidenced by an AE rate comparable to placebo, the majority of AEs having been reported as mild or moderate in severity, and the low withdrawal rate due to AEs (Supplementary Table II). No clinically important safety trends were observed post-dosing with GSK249320. There was no difference in the proportion of subjects having a fall, or in the number of falls, between treatment groups. The overall incidence of events common to stroke was comparable across the treatment groups (Supplementary Table III). Adverse events were reported in 57 (84%) of subjects in the placebo group, and 49 (75%) of subjects in the GSK249320 group. The most common AEs were constipation, nausea, and headache. No AE reports suggested peripheral neuropathy, infusion site reaction, or hypersensitivity reaction with GSK249320. Withdrawal from the study due to an AE occurred in two subjects in the placebo group and no subjects in the GSK249320 group.
Sixteen (24%) subjects in the placebo group experienced an SAE, compared to 9 (14%) in the GSK249320 group. Five (7%) subjects died in the placebo group. Two (3%) died in the GSK249320 group: respiratory failure in a 90-year old four days after first infusion, and cardiorespiratory arrest in a 76-year old 22 days after first infusion, both considered unrelated to IP.
Immunogenicity
Five subjects had pre-existing antibodies at low titers that were not related to treatment. Six of the 64 subjects who received GSK249320 developed anti-drug antibodies. Eight of the 68 subjects in the placebo treatment group had anti-drug antibodies against GSK249320 that were also not related to treatment. No neutralizing antibodies were detected.
GSK249320 reduced free serum MAG levels
Prior to administration of IP, soluble free MAG plasma levels were similar between placebo and GSK249320 groups (33.0±42.0 vs. 30.0±30.7 pg/ml, mean±SD). A progressive slow decline in free MAG level was seen after Day 6 for placebo subjects whereas subjects receiving GSK249320 exhibited an abrupt decline in free MAG level between Day 1 and 6 that was maintained until at least Day 30: median inhibition of free MAG in plasma was 97.5% after the first infusion of GSK249320 on Day 1 and was maintained after the second infusion on Day 6 at 97% until at least Day 30, with free MAG levels in GSK249320-treated subjects resuming to levels similar to placebo group subjects at Day 180 (Supplementary Figure I). The median GSK249320 concentration at the end of the second IP infusion, which can be considered the maximum concentration, was 494.5 mcg/ml, and the mean half-life of GSK249320 was 23.7±5.2 days (Supplementary Figure II).
Discussion
The current study hypothesized that GSK249320, administered as two IV infusions beginning 24–72 hours post-stroke and spaced 5±2 days apart, would improve gait recovery over 90 days in subjects with ischemic stroke and leg weakness with impaired walking ability. The data do not support this, and the study was stopped at interim analysis because observed difference between treatment groups met the predefined futility threshold.
The primary outcome measure was gait velocity, a choice that in retrospect had both advantages and disadvantages. Gait velocity has an established record as a valid, reliable assessment sensitive to treatment effects14, 15. Another advantage is that it measures function (i.e., disability, activities limitations), rather than impairment, and can be directly linked with participation level (i.e., handicap)15, 16, 18, 19. As a modality-specific outcome measure, gait velocity has potential advantages over global outcomes for understanding recovery such as granularity of assessment20. Furthermore, reduced gait velocity is common after stroke, gait improvements after stroke are linked to better quality of life, and in some studies gait recovery is ranked as the top priority by patients with hemiplegia after stroke16, 21, 22. The value of gait velocity as primary endpoint was also based in part on its direct link with entry criteria (Table 1), which required slow gait for study entry. However, at baseline, >80% of subjects were entirely unable to ambulate at all (gait velocity=0 m/sec), masking accurate understanding of within-subject gait recovery. This produced a floor effect such that several different degrees of neural abnormality were scored identically, although the study did make the key distinction between patients with gait velocity=0 m/sec and patients in whom gait velocity could not be assessed. Another potential disadvantage of gait as the primary endpoint is that it is a complex behavior influenced by activity at multiple nervous system levels. Many patients with severe hemiparesis learn to walk on their spasticity, further complicating interpretation of changes in gait velocity after stroke. Putting it in perspective, the current placebo group mean gait velocity change from baseline to day 90 (0.56 m/sec) was more than three-fold greater compared to placebo group of the prior phase II GSK249320 trial (0.18 m/sec)13, a difference possibly due to play of chance but that reduced ability of the current study to detect a treatment group difference. Level of impairment also differed between studies, with median placebo group baseline total NIHSS score of 7 in the prior trial compared to 9.5 herein.
Other study design features may also be important for understanding results. Choice of patient population influences how hypotheses are tested. Patients with small vessel infarcts, operationally less than 15 mm maximum diameter or 4cc volume23, were excluded given their comparatively favorable prognosis24, 25. Study entry required total NIHSS score 3–21 and leg motor score 1–4. This enrolled subjects with milder strokes, who might be expected to have a favorable prognosis regardless of treatment arm. The amount of IP infused could also be important. Median GSK249320 concentration at end of the second infusion (maximum concentration) was lower herein as compared to subjects receiving the same dose in the prior study20 in which the second infusion was administered 9±1 days apart (median 494.5 versus 723.0 mcg/ml); conceivably infusing a higher amount of antibody might have increased its effect size.
It is useful to revisit assumptions that supported current study design. The antibody showed a favorable preclinical and clinical profile. It was well characterized, and the progression of therapy development conformed to published recommendations17. Preclinical studies in rodents10 and primates11 suggested efficacy. The antibody was found to be safe in 37 healthy subjects, who received a single IV infusion up to 25 mg/kg12, and in a phase II study of 42 patients 24–72 hours after ischemic stroke, among whom 25 subjects received two IV infusions up to 15 mg/kg13; significant benefit over placebo was found over time for gait velocity, an endpoint well aligned with preclinical behavioral endpoints.
Other issues relevant to current results pertain to translation from animals to humans. Behavioral recovery26, 27 and neural plasticity28–30 after stroke are accelerated in rodents compared to humans. On this basis, time of first infusion in animals (24 hours post-stroke) was extended to 72 hours in humans, but this may not have been an appropriate extrapolation. The same concern might extend to presence of MAG, the biological target: in rats with experimental stroke, MAG levels start to increase by 3 days post-stroke and peak at 2 weeks post-stroke2 but it is uncertain whether this is true in humans. White matter constitutes 14% of rodent vs. 50% of human brain volume31, 32; axons might be more difficult for a large antibody to access in humans. Other limitations of animal models may also pertain, including that animal models incompletely capture the complex psychosocial issues patients face after stroke such as depression, caregiver support, and financial stressors33.
Direct evidence that substantial quantities of the therapy reached the biological target was not available. Indirect evidence of target binding in the current study was suggested by the substantial reduction in free MAG plasma levels with GSK249320 treatment. The half life of GSK249320 in the current study was 23.7±5.2 days, similar to the value of 21 days found in healthy control subjects and typical of a monoclonal antibody12. Neutralizing antibodies were not detected and so did not contribute to current findings.
The experience of translating therapies targeting acute ischemic stroke has provided a number of lessons34 and in many cases these inform translation of restorative stroke therapies to clinical trials. Examples include stepwise translation from preclinical to clinical studies, the need to standardize performance of assessments, careful selection of study sample size to insure adequate study power, and centralized data management. However, neuroprotection differs in many ways from restoration--restorative trials are not simply delayed neuroprotection trials. On the contrary, trials targeting brain restoration must address unique aspects of study design issues33 within the context of topics such as endpoint selection, target population identification, and intervention timing because the optimal approach in these and other areas often does not directly extend from neuroprotection trials to restorative trials1, 35.
This proof of concept study for GSK249320, a monoclonal antibody GSK249320 administered IV and initiated within 72 hours of stroke onset, demonstrated no improvement on gait outcomes compared to placebo. As above, a number of possible reasons might have contributed to these findings, including using an endpoint with too large a floor effect at baseline, enrolling patients with too severe a level deficit, using too low an antibody dose, inter-species differences in pharmacokinetics, lack of direct evidence that the therapy reached the biological target, or simply that GSK249320 does not work in human stroke. In the current study, the antibody was well tolerated and showed low immunogenicity, findings that may prove useful to future studies aiming to use a monoclonal antibody to modify activity in specific biological targets to promote improved stroke recovery.
Supplementary Material
Acknowledgments
Sources of funding
Steve Cramer is supported by NIH K24 HD074722. This study was supported by GlaxoSmithKline.
Footnotes
Conflict of interests
SCC has served as a consultant for GlaxoSmithKline, Roche, Dart Neuroscience, MicroTransponder, and RAND Corporation. LAE and MS are employees of GlaxoSmithKline and own shares in the company. CKR and TRT are former employees of GlaxoSmithKline and own shares in the company.
References
- 1.Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurology. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 4.Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. Journal Neurological Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Walmsley AR, Mir AK. Targeting the nogo-a signalling pathway to promote recovery following acute cns injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- 6.Irving EA, Vinson M, Rosin C, Roberts JC, Chapman DM, Facci L, et al. Identification of neuroprotective properties of anti-mag antibody: A novel approach for the treatment of stroke? J Cereb Blood Flow Metab. 2005;25:98–107. doi: 10.1038/sj.jcbfm.9600011. [DOI] [PubMed] [Google Scholar]
- 7.Torigoe K, Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Experimental neurology. 1998;150:254–262. doi: 10.1006/exnr.1997.6775. [DOI] [PubMed] [Google Scholar]
- 8.Mears S, Schachner M, Brushart TM. Antibodies to myelin-associated glycoprotein accelerate preferential motor reinnervation. J Peripher Nerv Syst. 2003;8:91–99. doi: 10.1046/j.1529-8027.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cash DEA, Mesquita M, Beech J, Williams S, Lloyd A, Irving E, et al. GSK249320, a monoclonal antibody against the axon outgrowth inhibition molecule myelin-associated glycoprotein, improves outcome of rodents with experimental stroke. Journal of Neurology & Experimental Neuroscience. in press. [PMC free article] [PubMed] [Google Scholar]
- 11.Barbay S, Plautz EJ, Zoubina E, Frost SB, Cramer SC, Nudo RJ. Effects of postinfarct myelin-associated glycoprotein antibody treatment on motor recovery and motor map plasticity in squirrel monkeys. Stroke. 2015;46:1620–1625. doi: 10.1161/STROKEAHA.114.008088. [DOI] [PubMed] [Google Scholar]
- 12.Abila B, Cunningham E, Simeoni M. First-time-in-human study with gsk249320, a myelin-associated glycoprotein inhibitor, in healthy volunteers. Clin Pharmacol Ther. 2013;93:163–169. doi: 10.1038/clpt.2012.227. [DOI] [PubMed] [Google Scholar]
- 13.Cramer SC, Abila B, Scott NE, Simeoni M, Enney LA. Safety, pharmacokinetics, and pharmacodynamics of escalating repeat doses of gsk249320 in patients with stroke. Stroke. 2013;44:1337–1342. doi: 10.1161/STROKEAHA.111.674366. [DOI] [PubMed] [Google Scholar]
- 14.Richards C, Malouin F, Dumas F, Tardif D. Gait velocity as an outcome measure of locomotor recovery after stroke. In: Craik R, Oates C, editors. Gait analysis: Theory and application. St. Louis: Mosby; 1995. pp. 355–364. [Google Scholar]
- 15.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. New Engl J Med. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz S, Lusardi M. White paper: “Walking speed: The sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 17.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Physical therapy. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 19.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 20.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38:1393–1395. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 21.Salbach NM, Mayo NE, Higgins J, Ahmed S, Finch LE, Richards CL. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Archives PMR. 2001;82:1204–1212. doi: 10.1053/apmr.2001.24907. [DOI] [PubMed] [Google Scholar]
- 22.Bohannon R, Andrews A, Smith M. Rehabilitation goals of patients with hemiplegia. Int J Rehab Research. 1988;11:181–183. [Google Scholar]
- 23.Fisher CM. Lacunar strokes and infarcts: A review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 24.Pullicino P, Nelson RF, Kendall BE, Marshall J. Small deep infarcts diagnosed on computed tomography. Neurology. 1980;30:1090–1096. doi: 10.1212/wnl.30.10.1090. [DOI] [PubMed] [Google Scholar]
- 25.Sprigg N, Gray LJ, Bath PM, Lindenstrom E, Boysen G, De Deyn PP, et al. Early recovery and functional outcome are related with causal stroke subtype: Data from the tinzaparin in acute ischemic stroke trial. J Stroke Cerebrovasc Dis. 2007;16:180–184. doi: 10.1016/j.jstrokecerebrovasdis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Kawamata T, Dietrich W, Schallert T, Gotts J, Cocke R, Benowitz L, et al. Intracisternal basic fibroblast growth factor (bFGF) enhances functional recovery and upregulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci. 1997;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan P, Goldstein L, Matchar D, Divine G, Feussner J. Measurement of motor recovery after stroke. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 28.Dijkhuizen R, Ren J, Mandeville J, Wu O, Ozdag F, Moskowitz M, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calautti C, Leroy F, Guincestre J, Baron J. Dynamics of motor network overactivation after striatocapsular stroke: A longitudinal pet study using a fixed-performance paradigm. Stroke. 2001;32:2534–2542. doi: 10.1161/hs1101.097401. [DOI] [PubMed] [Google Scholar]
- 30.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher J, Tardy J, et al. A longitudinal fmri study: In recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–332. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- 32.Cramer S. Clinical issues in animal models of stroke and rehabilitation. ILAR Journal. 2003;44:83–84. doi: 10.1093/ilar.44.2.83. [DOI] [PubMed] [Google Scholar]
- 33.Cramer SC. Drugs to enhance motor recovery after stroke. Stroke. 2015;46:2998–3005. doi: 10.1161/STROKEAHA.115.007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirnagl U. Thomas willis lecture: Is translational stroke research broken, and if so, how can we fix it? Stroke. 2016;47:2148–2153. doi: 10.1161/STROKEAHA.116.013244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.