Abstract
Bisindole natural products consist of two monomeric indole alkaloid units as their obligate constituents. Bisindoles are more potent with respect to their biological activity than their corresponding monomeric units. In addition, the synthesis of bisindoles are far more challenging than the synthesis of monomeric indole alkaloids. Herein is reviewed the enantiospecific total and partial synthesis of bisindole alkaloids isolated primarily from the Alstonia genus of the Apocynaceae family. The monomeric units belong to the sarpagine, ajmaline, macroline, vobasine, and pleiocarpamine series. An up-to-date discussion of their isolation, characterization, biological activity as well as approaches to their partial and total synthesis by means of both synthetic and biosynthetic strategies are presented.
Keywords: Alstonia genus, Apocynaceae family, sarpagine, macroline, ajmaline, bisindole alkaloids, biomimetic synthesis, partial and total synthesis, enantiospecific and regiospecific synthesis of alkaloids, bioactive indole alkaloids
1. Introduction
Indole alkaloids are of extraordinary significance due to their structural diversity, medicinal properties and are an essential part of the drug discovery process (see the Merck Index for details) [1]. Their medicinal values are evident from folklore and their ethnopharmacological uses worldwide stem from ancient times [2–5]. Many of the alkaloids are marketed drugs [6], and other drugs are derived directly from natural products or they serve as lead compounds for further development [7] into clinical candidates ranging from anti-bacterial to anti-cancer agents [8–15].
The activity and structures of bisindole alkaloids have been reviewed and the number of isolated bisindole alkaloids are increasing [16–19]. This review is focused on the partial and total synthesis of bisindoles, as well as some dimeric indole alkaloids wherein at least one of the following monomeric units contains, as its obligate constituent, the sarpagine, macroline, ajmaline, vobasine, or pleiocarpamine systems. Because of the complexity of their structures, which are comprised of more than one alkaloidal partner, a convergent approach toward their synthesis is logical synthetically, as well as biosynthetically. This breaks down to synthesis of the monomeric alkaloidal units, followed by a biomimetic-type condensation to arrive at the bisindole framework. The number of isolated bisindoles has increased recently because of modern spectroscopic techniques (e.g., Mass spectrometry, 13C and 2D NMR, HMQC, HMBC, NOESY, and X-ray crystallography) which enable accurate structure determination on milligram quantities of the bisindole. The synthesis of these complex natural products can begin only when their structures are accurately confirmed [20]. Consequently, the synthesis of bisindole alkaloids, of course, must lag behind the synthesis of monomers which comprise them. An important approach, defined as partial synthesis, is to combine any monomeric alkaloid available, either by synthesis (including the synthesis from a natural product) or by isolation, with the other natural monomeric unit usually in a biosynthetic strategy. This process is commonly termed the biomimetic synthesis of bisindole alkaloids pioneered by Schmid, LeQuesne, Kutney, Magnus, and others.
In many studies, it has been shown that bisindole alkaloids exhibit more potent biological, pharmacological and medicinal properties than the corresponding monomers which comprise them [21,22], as mentioned above. There are many theories as to why this pattern of activity is observed but, to date, no concrete evidence as to the mechanism of action (MOA) has emerged. However, this is not surprising for one would not expect the same MOA in each case. As always one of the main hurdles in natural product related drug-discovery is that many natural products are present in very small quantities and cannot be isolated in quantities high enough to study their biological activity let alone structure-activity relationships. This situation is exacerbated in the case of bisindole alkaloids. Consequently, it is essential to develop better and easier access to bisindole alkaloids either synthetically or biosynthetically. Partial and total synthesis, thereby require equal emphasis in this regard. If one can synthesize the natural products in a stereospecific, regiospecific and enantiospecific sense via a practical synthetic route, these alkaloids could be obtained in appropriate amounts for detailed studies of their biological activity. One could then condense the mismatched pairs as well (i.e., (+)(−); (+)(+); (−)(−); (−)(+)).
The discovery of the Vinca alkaloids vinblastine and vincristine, originally isolated from Madagascar periwinkle, Catharanthus roseus (formerly known as Vinca rosea) [23–26], marked a major milestone in natural product-based drug development as these two are the first clinically used alkaloidal anti-cancer agents. Moreover, it is well known that the bisindole nature of these alkaloids is the key for the bisindoles are much more active than the monomeric alkaloids which comprise them. These bisindole (indole-indoline) alkaloids are one of the best treatment options in various types of cancers and have been successfully used in combination therapies since the early 1960s [27–29]. Besides, their MOA (interaction with tubuline, microtubule assembly inhibition, and mitosis inhibition) is one of the most effective treatment against cancer, which promoted rigorous research and development of vinblastine and vincristine resulting in many (derivatives of vincristine and vinblastine) clinical candidates to date [6,8–15,30–36]. As a consequence, these have profoundly stimulated the search and development of alkaloidal natural product based drugs.
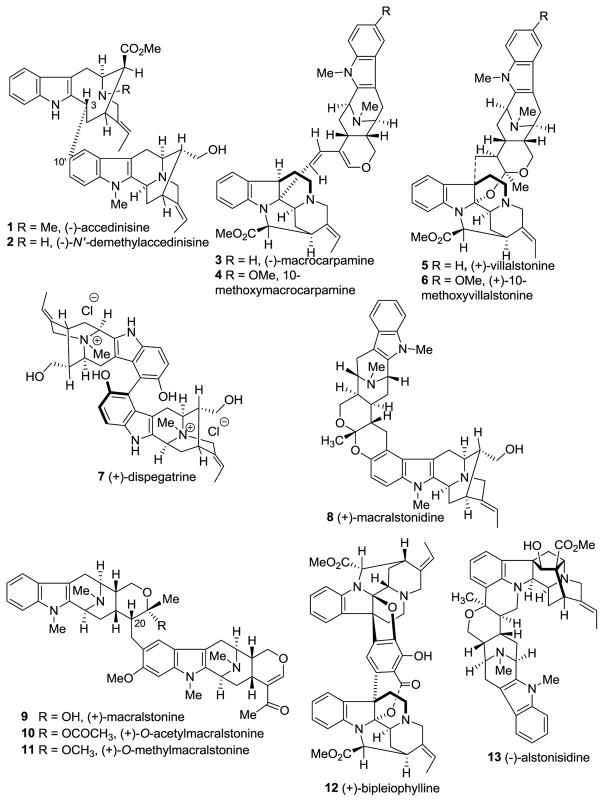
The bisindoles (−)-accedinisine 1 and (−)-N′-demethylaccedinisine 2 exhibit anti-leishmanial activity, as well as moderate antibacterial activity against gram-positive B. subtilis [37,38]. Antimalarial activity of numerous indole alkaloids against Palsmodium falciparum (cerebral) malaria have been reported, many of which are bisindole alkaloids [21,39–41]. (−)-Macrocarpamine 3 and (+)-villalstonine 5 were the most active bisindoles isolated from Alstonia macrophylla against both Entamoeba histolytica and Plasmodium falciparum [21,42]. These alkaloids 3 and 5 also exhibited potent anti-cancer activity against lung cancer cell lines as well as melanoma, renal cell carcinoma, breast, and colon adrenocarcinoma [22,40]. Villalstonine 5 was also active against murine leukemia cell lines [43]. (+)-Dispegatrine 7 and its related monomer, (+)-spegatrine exhibited potent hypotensive activity, wherein the potency of the dimer was about an order of magniture greater than the monomer toward α1 and α2 adrenergic receptors [44]. (+)-Macralstonine 9 has been reported to have hypotensive activity [45,46]. In another study, by Keawpradub et al. on three Alstonia species from Thailand, bisindoles (+)-macralstonine 9, (+)-O-acetylmacralstonine 10, (+)-O-methylmacralstonine 11, (+)-villalstonine 5, and (−)-macrocarpamine 3, as well as the monomeric alkaloids (+)-pleiocarpamine 14, (−)-alstonerine 15, and (−)-alstophylline 22 exhibited profound anti-Plasmodial activity [47]. It is important to note that a minor change in the structure of (+)-macralstonine 9 by conversion into the O-methyl ether 11 or O-acetyl ester 10, profoundly increased its anti-Plasmodial activity [47]. This increase in activity of O-acetyl and O-methyl derivatives of macralstonine may be due to the increased lipophilicity which facilitated their transport across cell membranes. Accedinisine-related monomeric indole alkaloids (+)-pericyclivine (92, see later), (−)-perivine (43, see later) and (−)-vobasine (not shown) exhibited significant cytotoxicity against P388 and KB cells but were less active than the bisindole alkaloids [20,48]. (+)-Pleiocarpamine 14 has recently been found to form an unprecedented dimeric structure, (+)-bipleiophylline 12, wherein two pleiocarpamine 14 units were linked together by means of an aromatic spacer (pyrocatechuic acid). This dimer was found to be very effective against drug-sensitive, vincristine resistant human KB and Jurkat cells [49]. This finding also supports the argument that bisindole alkaloids are more active than their progenitors since (+)-pleiocarpamine did not exhibit significant activity [21,22,47].
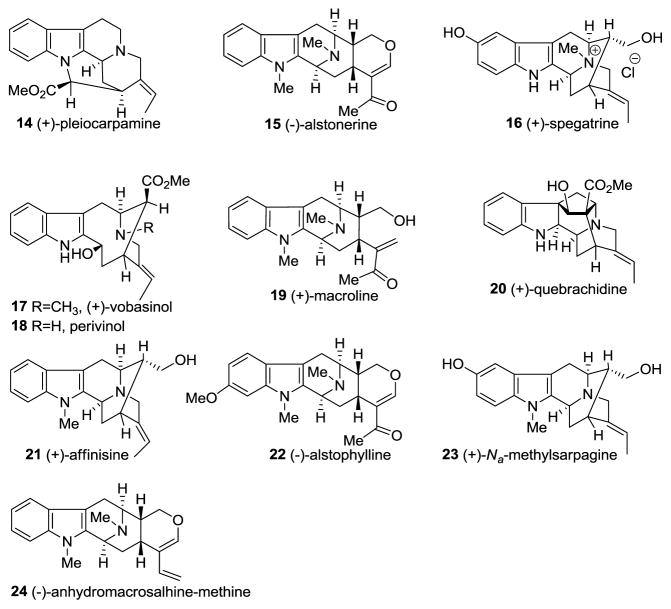
Illustrated in Figure 1 are representative bisindoles presented in this review. All of these alkaloids have originated from the combination of two monomeric indole alkaloids depicted in Figure 2.
Figure 1.
Representative examples of bioactive bisindole alkaloids.
Figure 2.
Monomeric units present in the bisindole alkaloids reviewed herein.
2. Biogenetic Relationship between Sarpagine/Macroline/Ajmaline Alkaloids
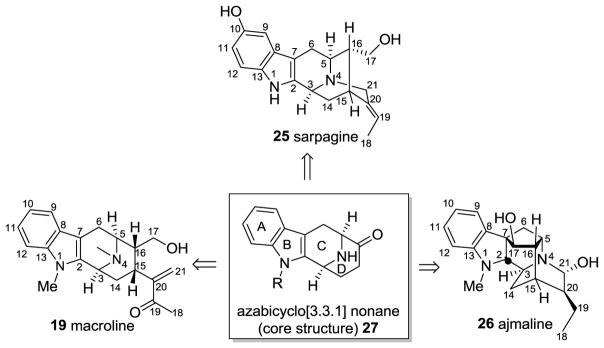
The macroline 19 and sarpagine 25 natural products are contained in one of the major classes of indole alkaloids that originated from common biogenetic intermediates. Although macroline 19 has not been isolated as an alkaloid itself, it is believed to be a biogenetic precursor for numerous alkaloids in this class. (+)-Ajmaline 26, a clinically and historically important alkaloid [20,50–52], is structurally related to the sarpagine alkaloids. These three major types of indole alkaloids are inter-related through their biogenetic origin. To date, this group consists of more than 300 alkaloids which contain more than 80 bisindole alkaloids [53,54]. A general strategy has been developed to access a common intermediate to this series of natural products, the tetracyclic core of which consists of the azabicyclo[3.3.1]nonane system with the (S) configuration at both C-3 and C-5 (Figure 3).
Figure 3.
The sarpagine, macroline, and ajmaline indole alkaloids. The biogenetic numbering of LeMen and Taylor has been followed [55].
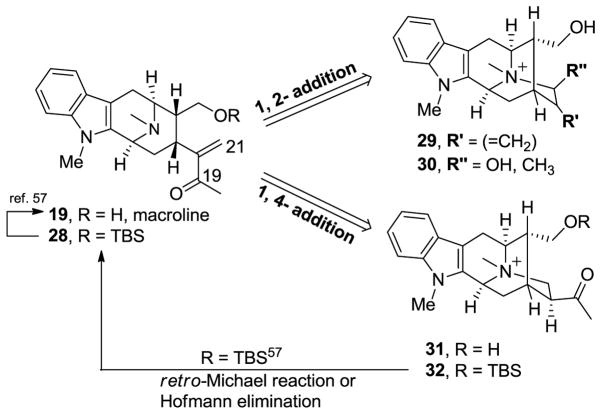
In a synthetic sense, sarpagine alkaloids could originate from a Michael-type reaction of the N(4) nitrogen atom of macroline 19 onto the α,β-unsaturated carbonyl system at C-21 either by a 1,2 or a 1,4-type addition reaction, as illustrated in Figure 4 [54,56]. On the other hand, the sarpagine 32 can be converted into macroline 19 by a retro-Michael process, reported in a biomimetic sense originally by LeQuesne et al. [57].
Figure 4.
Interconversion of macroline 19 and sarpagine 25.
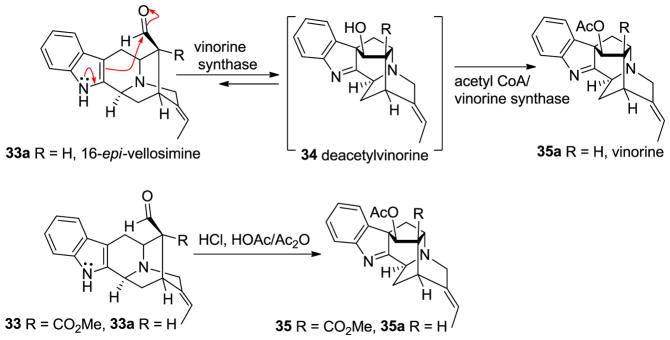
The biogenetic relationship between these series of alkaloids has been reported previously [58–61]. Stöckigt et al. converted 16-epi-vellosimine 33a into vinorine 35a via deacetylvinorine 34 using acetyl CoA dependent vinorine synthase [62–64]. This study confirmed the conversion of the endo-aldehyde containing sarpagine skeleton (33) into the ajmaline skeleton (35) under acidic conditions, as suggested earlier by Woodward (Figure 5) [58].
Figure 5.
Biosynthetic relationship between sarpagine and ajmaline reported by Stöckigt et al. [62–64].
3. General Strategy to Access the Tetracyclic Core 27 of the Sarpagine/Macroline/Ajmaline Related Indole Alkaloids
An azabicyclo[3.3.1]nonane core system 27 is a common feature of the macroline/sarpagine/ajmaline type indole alakaloids. Thus, a general synthetic strategy for the synthesis of these alkaloids involved the synthesis of this core structure with the correct stereochemistry at positions C-3, C-5 and appropriate functional groups at C-15 and C-16 for further functionalization. To date, methods for accessing the (±)-azabicyclo[3.3.1]nonane 27 system has been reported by Yoneda [65], Mashimo [66], Kluge [67] and on large scale by Soerens et al. [68].
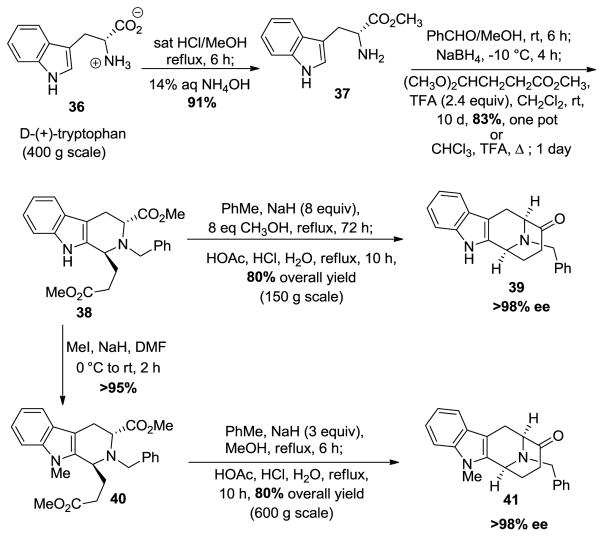
The synthesis of the optically active (−)-tetracyclic core was achieved by Zhang et al. [69] in 1988 in a stereospecific fashion which employed the asymmetric Pictet-Spengler reaction. Numerous adjustments have been made to better access this moiety with both Na-H and Na-Me functionalities over the years. The present strategy is illustrated in Scheme 1. Currently, the Pictet-Spengler reaction is carried out in one pot under conditions that permit epimerization at C-1 to provide only the desired trans-diastereomer 38. D-(+)-tryptophan methyl ester 37 is commercially available but can also be prepared from D-(+)-tryptophan 36 by Fischer esterification on 400 gram scale.
Scheme 1.
General strategy to access the tetracyclic ketones which contain the azabicyclo[3.3.1]nonane system.
The Nb-benzylation was done on amine 37 with benzaldehyde in methanol at room temperature, followed by reduction of the so formed iminium ion salt with sodium borohydride added slowly to the mixture at −10 °C. Subsequently, trifluoroacectic acid was added to the mixture at 0 °C to bring the pH to 7. After removal of the solvent, methylene chloride, trifluoroacetic acid (TFA) and 4,4-dimethoxybutyrate were added to the mixture at 0 °C and this was stirred for 10 days at room temperature to yield the trans-diester 38 in 83% yield as the sole product [70]. This could be accomplished in refluxing chloroform in one day in slightly lower yield. Next, the Dieckmann cyclization was done in the presence of a large excess of NaOMe and methanol in toluene at reflux for 72 h (6 h for the Na-Me indole) to yield the cyclized product. At the completion of the reaction, the reaction mixture was cooled to 0 °C and the excess base was neutralized with glacial acetic acid and the solvent was removed under reduced pressure. After that, glacial acetic acid and aqueous hydrochloric acid as well as water were added to the mixture and it was allowed to reflux under these strongly acidic conditions to furnish the product of decarboxylation. This gave Nb-benzyl tetracyclic ketone 39, in 80% overall yield on large scale. For the Na-Me series, the trans-diester 38 was methylated using iodomethane and sodium hydride in DMF to yield the Na-Me trans-diester 40 in >95% yield, which under the same conditions as 38 gave the Na-Me tetracyclic ketone 41 in a one-pot process. This route is still one of the best routes since reactions can be done on multi-hundred gram scales and do not require protection of the Na-H function. This is an enantioselective and regioselective process in >98% ee. This route with two one pot conversions also requires fewer steps than previous routes [69–71]. In addition, the availability of D-(+)-tryptophan and the (−)-enantiomer provides a route to both enantiomers of the alkaloids including the ability to condense mismatched pairs, as mentioned.
4. Partial and Total Synthesis of Bisindole Alkaloids
4.1. (−)-Alstonisidine
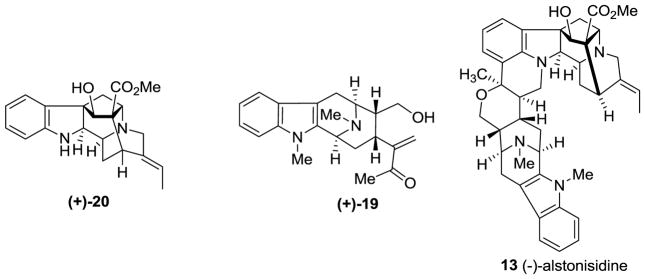
(−)-Alstonisidine was isolated from the bark of Alstonia muelleriana Domin, along with several other alkaloids by Elderfield et al. and LeQuesne et al. [72,73]. The structure of (−)-alstonisidine 13 was reported in 1972 by LeQuesne et al. [74,75]
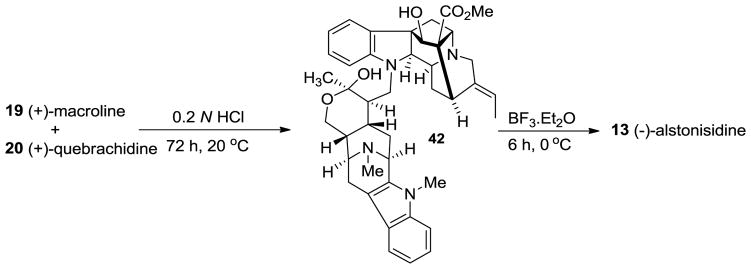
The structure of 13 was proposed, in a biomimetic sense, for (−)-alstonisidine from its constituent monomeric units, (+)-quebrachidine 20 and (+)-macroline 19. The crystal structure of (−)-alstonisidine was ultimately reported by Hoard in 1977 which confirmed the structure as 13 (Figure 6) [76]. When the mixture of 19 and 20 was stirred in 0.2 N aqueous HCl, the labile hemiketal 42 formed, as indicated by examination of the spectral data. The tentative stereochemistry was based on the structure of the bulky quebrachidine unit which bore a–CH2–group which should occupy the equatorial position. The hemiketal was treated with boron trifluoride etherate to yield alstonisidine which suggested the structure 42 as an intermediate, since no reaction occurred under the same conditions between (+)-quebrachidine 20 and (+)-macroline 19 [75].
Figure 6.
Structures of (+)-quebachidine (20), (+)-macroline (19), and (−)-alstonisidine (13).
This biomimetic synthesis of (−)-alstonisidine 13 employed the biogenetic precursors (+)-macroline 19 and (+)-quebrachidine 20. Although macroline has never been isolated as a natural product, it is widely agreed to be a biogenetic precursor for (−)-alstonerine [77], (−)-alstophylline [78], and (+)-alstonisine [72] and may be present in very small steady-state concentrations in plants. Furthermore, the isolation of traces of (+)-quebrachidine from A. muelleriana further strengthened this proposed biogenetic route. (+)-Quebrachidine 20 has been isolated from Vinca libanotica [79], Ochrosia acuminate [80], Alstonia muelleriana [77], and the leaves of Aspidosperma quebracho blanco Schletch [81] and its structure was proposed to be 20 based on the studies by NMR and mass spectroscopy [82]. Four aromatic proton multiplets (δ = 6.6 to 7.2) indicated it contained an unsubstituted aromatic ring, accompanied by an ethylidene group. Examination of the Infrared spectrum indicated the presence of N-H (3590 cm−1), O-H (3370 cm−1) and a carbomethoxyl (1722, 1235 cm 1 and 1H singlet at δ = 3.6) group. Upon acetylation, the compound gave a diacetate. Analysis of the NMR, IR and UV spectra of the diacetate indicated the presence of a secondary alcohol and a dihydroindole nitrogen atom. Analysis of the mass spectrum further strengthened the proposed structure of (+)-quebrachidine to be 20 [82].
In the biomimetic partial synthesis of (−)-alstonisidine 13 [83], (+)-macroline 19 was prepared by degradation of villamine (not shown) by the method of Schmid et al. [84]. (+)-Macroline 19 reacted with naturally occurring (+)-quebrachidine 20 under mildly acidic conditions at 20 °C (Scheme 2). Initially macroline underwent a Michael reaction with the indoline nitrogen atom of quebrachidine and subsequently underwent intramolecular cyclization to form the amino hemiketal 42. This hemiketal species was found to be highly labile to acid since it reversed to the starting materials 19 and 20 upon warming, as well as under stronger acidic conditions and during chromatography. Fortunately, no other compounds were observed except the starting materials 19 and 20. The new indole 42 had a molecular weight of 690 and examination of the IR spectrum indicated the presence of only one carbonyl peak (1735 cm−1) which indicated that after the initial 1,4-conjugate addition had occured, the so formed ketone on the macroline unit reacted intramolecularly with the–CH2OH group to form the corresponding hemi-ketal present in 42. When hemiketal 42 was treated with boron trifluoride at 0 °C for 6 h, the electrophilic aromatic substitution took place, ortho to the amino group of quebrachidine to form the tetrahydropyran which was indistinguishable from the naturally occurring alstonisidine 13 (Scheme 2) from A. muelleriana [77,83].
Scheme 2.
Biomimetic partial synthesis of (−)-13 by LeQuesne et al [75].
The total synthesis of (−)-alstonisidine has not been reported yet. Extensive work has been done toward its monomeric constituents 19 and 20. To date, The Milwaukee group has achieved the total synthesis of (+)-macroline 19 [85–88] and the enantiomer of macroline as well [89]. Besides an advanced intermediate for 20, the synthesis of quebrachidine diol (not shown) has been reported as well [90]. The partial synthesis of (−)-alstonisidine has been completed, to date, from synthetic (+)-macroline 19 and natural (+)-quebrachidine 20.
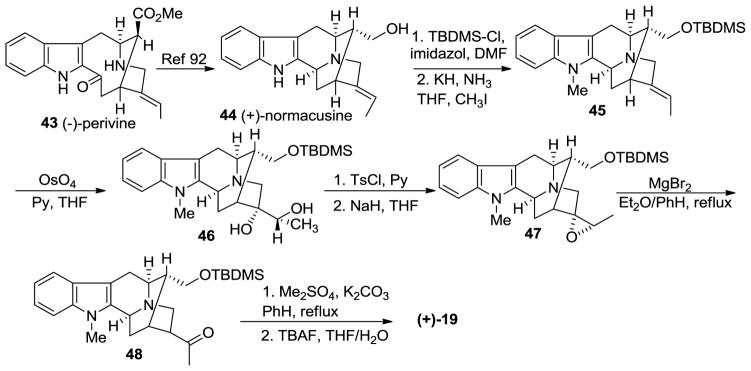
The total synthesis of bisindole 13 awaits the total synthesis of 20. The earliest synthesis of (+)-macroline 19, a biomimetic synthesis, was done by LeQuesne et al. in 1980 [57] and the absolute configuration had been confirmed by X-ray crystallography by Hesse in 1981 [91]. In this synthesis, LeQuesne et al. converted (−)-perivine 43 to (+)-normacusine 44, according to the reported procedure by Gorman et al. (Scheme 3) [92]. The protection of the hydroxyl group (in 44) as the tert-butyldimethylsilyl ether, was followed by Na-methylation to furnish the sarpagine system 45. Osmylation of olefin 45 with OsO4 and pyridine in THF gave an inseparable mixture which contained the diol 46, along with a related spirooxindole byproduct. The bulky TBDMS function rendered the osmylation sequence stereoselective, although marred by the formation of the spirooxindole side-product. This mixture was treated with TsCl and Py, followed by NaH in THF to give the epoxide 47 that could be purified by crystallization. The epoxide underwent rearrangement on treatment with freshly prepared MgBr2 to furnish the ketone 48. The methylation of the Nb function was performed with dimethyl sulfate and potassium carbonate, in refluxing benzene. This was followed by the retro-Michael reaction in base followed by desilylation with TBAF to provide (+)-macroline 19 [57].
Scheme 3.
Biomimetic partial synthesis of (+)-19 by LeQuesne et al [57].
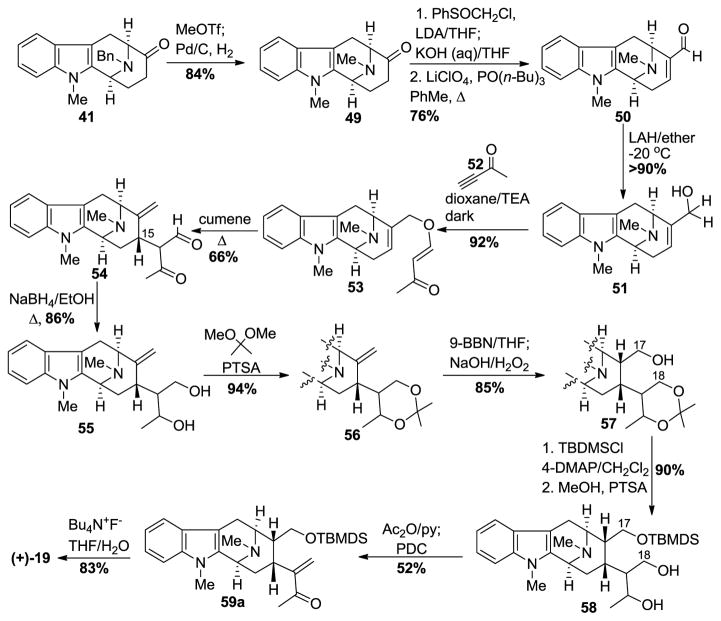
In an enantiospecific synthetic approach to (+)-macroline [85,86], the readily available Nb-benzyl tetracyclic ketone [93–95] intermediate 41, available on 300 gram scale, was converted into the corresponding Nb-methyl indole compound 49 by methylation with methyl triflate followed by catalytic debenzylation to form the Nb-methyl system in 84% yield (Scheme 4). The ketone 49 was reacted with the anion of chloromethyl phenyl sulfoxide (LDA, THF, −78 °C) to generate the corresponding chlorohydrin. This chlorohydrin, upon stirring in a heterogeneous solution of 10 N KOH in THF at 25 °C for 24 h produced the corresponding spirooxirane and this was followed by treatment with LiClO4 and PO(n-Bu)3 in refluxing toluene to produce the α,β-unsaturated aldehyde 50 [96]. Chemo-selective reduction of the aldehyde with lithium aluminum hydride in ether at −20 °C gave the allylic alcohol 51. Michael addition of the alcohol to 3-butyn-2-one 52 provided the desired enone ether 53 in 92% yield. The Claisen rearrangement of the enone ether produced the β-dicarbonyl compound 54, which was subsequently reduced with sodium borohydride to give the corresponding diol 55. The 1,3-diol functionality was protected as an acetonide 56 using acetone dimethyl acetal in the presence of p-toluenesulfonic acid. The hydroboration of the olefin in 56 with 9-BBN occurred in a stereospecific fashion and this was followed by oxidation with a basic solution of hydrogen peroxide, under classical conditions, to furnish the C-17 primary alcohol 57. The hydroxyl group of monol 57 was protected as the t-butyldimethylsilyl ether, and subsequent selective removal of the acetonide was done using p-toluenesulfonic acid in dry methanol to provide diol 58. To access the α,β-unsaturated carbonyl system in macroline, it was planned to use the tosylate of the C-18 hydroxyl group as the leaving group. But the tosylation with tosyl chloride and pyridine in DCM was not successful, and mesylation took place with very poor regioselectivity. Based upon a precedent by Helquist et al. [97], acetylation with acetic anhydride worked both as a protecting group and leaving group. The so formed monoacetate was subjected to PDC oxidation. The acetate group underwent elimination spontaneously upon oxidation to provide the stable TBDMS protected macroline equivalent 59a, which could be stored in a vial. The silyl ether was deprotected with tetrabutylammonium fluoride in THF to provide (+)-macroline 19 identical to the natural material obtained from villamine by Schmid et al. [84].
Scheme 4.
Enantiospecific total synthesis of (+)-19 by Bi et al [85].
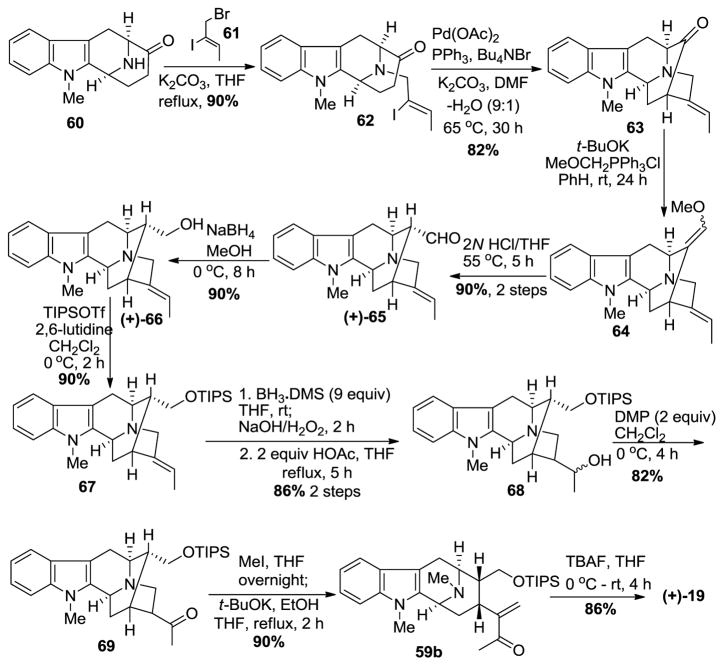
In another more recent and improved approach (Scheme 5) [87,88], Nb-alkylation via SN2 substitution on (Z)-1-bromo-2-iodo-2-butene 61 in the presence of K2CO3 in refluxing THF gave the vinyl iodide 62. The palladium catalyzed intramolecular cyclization of the enolate anion with the vinyl iodide took place in a stereospecific manner to yield the pentacyclic ketone 63. A one-carbon homologation via Wittig olefination with methoxymethyl triphenylphosphonium chloride with potassium tert-butoxide in benzene, followed by hydrolysis of the so formed enol ether 64 in aqueous HCl/THF furnished the α-aldehyde 65 ((+)-Na-methylvellosimine; an alkaloid isolated from Alstonia nitida [98]). The aldehyde was reduced with sodium borohydride to provide (+)-affinisine 66, another alkaloid obtained from A. macrophylla [99]. The protection of the primary alcohol as the TIPS ether with triisopropylsilyl triflate and 2,6-lutidine gave indole 67. The hydroboration-oxidation of the olefin in 67 went smoothly to provide the secondary alcohol 68 in very good yield, accompanied by a small amount of the corresponding tertiary alcohol. An equivalent of BH3 is known to complex with the Nb-nitrogen function which alters the electronic character in the vicinity of the olefin, which presumably resulted in the formation of the small amount of tertiary alcohol. The Dess-Martin periodinane-mediated oxidation of the alcohol 68 to the ketone 69 took place in high yield. The quaternization of the Nb-nitrogen with methyl iodide in THF at 0 °C, was followed by treatment of the so formed Nb-methiodide salt with potassium tert-butoxide in ethanol under modified conditions to provide the stable macroline equivalent 59b. Again, this intermediate can be easily stored. The de-silylation with tetrabutylammonium fluoride resulted in the synthesis of (+)-macroline 19 identical to that obtained above in much higher overall yield and fewer steps. These reactions could be scaled up with ease as well.
Scheme 5.
Improved total synthesis of (+)-macroline (19) by Liao et al [87].
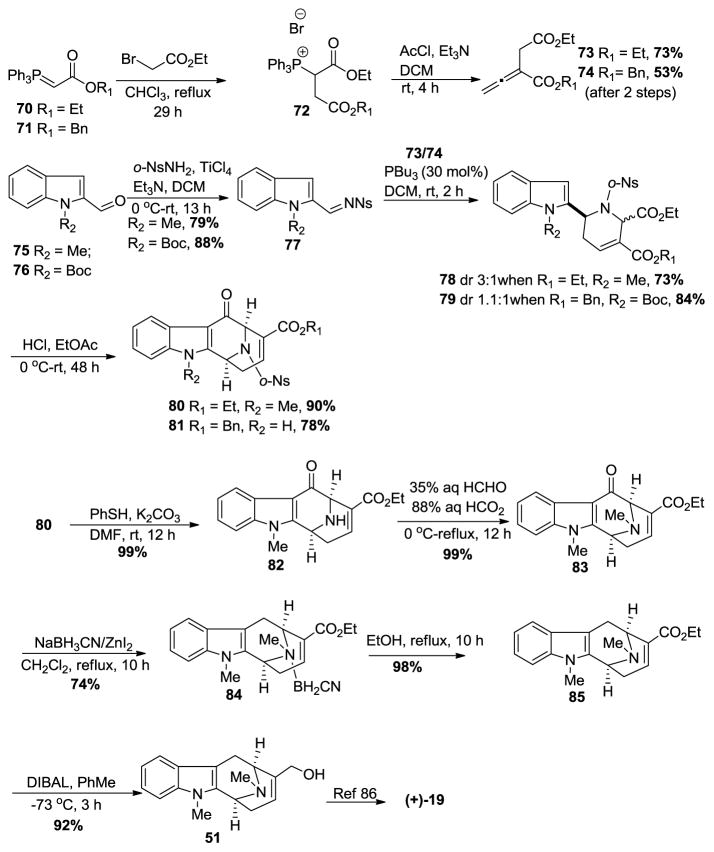
Kwon recently reported a formal synthesis of (+)-macroline 19 as well, which employed a phosphine-catalyzed [4 + 2] annulation process (Scheme 6) [100]. This route began from commercially available phosphorene 70 or 71; the first step of which was to generate the phosphonium salt 72 by reaction with the bromoacetate. Treatment of 72 with acetyl chloride in the presence of trimethylamine furnished the allene 73 or 74, individually. The condensation of o-nitrobenzenesulfonamide with either of the indole-2-carboxaldehydes 75 or 76 in the presence of TiCl4 and Et3N gave the imines 77 in very good yield, respectively. The allene (73 or 74) was then reacted with the imine 77 in the presence of tributylphosphine in methylene chloride to yield the [4 + 2] annulation products as inseparable mixtures with moderate diastereoselctivity (dr 3:1 when R1 = Et, R2 = Me and dr 1.1:1 when R1 = Bn, R2 = Boc). The C-5 carboxylic acid ester was epimerized under acidic conditions to facilitate the intramolecular Friedel-Crafts acylation to provide the bridged bicyclic compounds 80 or 81.
Scheme 6.
Formal synthesis of (+)-macroline (19) by Kwon et al [100].
The nosyl group on the Nb-nitrogen atom in 80 was removed by the method of Fukuyama [101] and proceeded smoothly to provide the Nb-H free base 82 in excellent yield (Scheme 6). An Eschweiler-Clarke reaction [102,103] gave the otherwise troublesome Nb-methylated product 83 in quantitative yield. The deoxygenation at C-6 under standard reductive conditions either did not react or gave the C-6 alcohol. However, the use of ZnI2-NaCNBH3, gave the desired aryl alkane as an Nb-complex 84 with cyanoborane, which upon heating in refluxing ethanol gave the free amine 85. Reduction of 85 with DIBAL in toluene gave the allylic alcohol 51, a known intermediate toward (+)-macroline 19 and (−)-alstonerine 15 previously reported [86] by The Milwaukee group and hence this completed a formal synthesis of (+)-macroline 19.
4.2. (−)-Accedinisine and (−)-N′-demethylaccedinisine
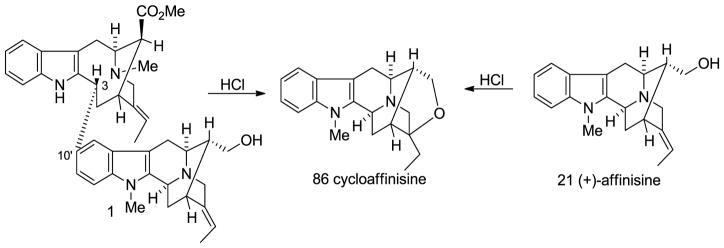
(−)-Accedinisine 1 was isolated along with several other bisindole alkaloids from Tabernaemontana accedens Muell. Arg. (Apocynaceae) and Peschiera van heurkii [38,104]. This dimeric indole alkaloid contained both a vobasine and a sarpagine unit. Upon treatment with hydrochloric acid, natural accedinisine 1 yielded cycloaffinisine 86 which could also be prepared from affinisine 21 under the same conditions. This clearly indicated the presence of an affinisine 21 unit in this dimeric alkaloid. Extensive studies of 1 by NMR and mass spectroscopy of the bisindole suggested the presence of a vobasinol unit as the remaining part of the molecule which was confirmed by the condensation of (+)-vobasinol 17 with (+)-affinisine 21 in aqueous HCl to yield a compound identical to natural (−)-accedinisine 1 (Scheme 7) [104]. Another vobasine-sarpagine (perivinol 18 + affinisine 21) bisindole alkaloid (−)-N′-demethylaccedinisine 2 (demethylaccedinisine) was isolated from Peschiera buchtieni [105] and Peschiera van heurkii [38]. The structure was confirmed by methylation of (−)-2 to yield the identical compound to the authentic sample of (−)-accedinisine 1 [105]. Both of this dimeric indole alkaloids have the constituent monomeric alkaloids linked from the C-3 position of the vobasine unit (vobasinol 17 or perivinol 18) to the C-10′ of the sarpagine unit (affinisine 21), as indicated by NMR spectroscopy and partial synthesis [104,105]. Both of these bisindoles are active against leishmaniasis. It is of interest that these alkaloids are purportedly active against only one of the strains of leishmaniasis that were studied indicating selectivity.
Scheme 7.
Formation of cycloaffinisine from (−)-accedinisine (1) and (+)-affinisine (21) under acidic conditions.
The vobasine portion of these alkaloids originated from C(3)-Nb disconnection of the sarpagine skeleton of (+)-peicyclivine (see later) and similar to the other unit, (+)-affinisine 21, could be accessed by a general and efficient approach from commercially available D-(+)-tryptophan methyl ester 37 (Scheme 8). Yang et al. achieved a total synthesis of (−)-accedinisine 1 and (−)-N′-demethylaccedinisine 2 [106–109].
Scheme 8.
Enantiospecific total synthesis of (+)-16-epi-vellosimine (33) and (+)-pericyclivine (92) by Yang et al [106,107].
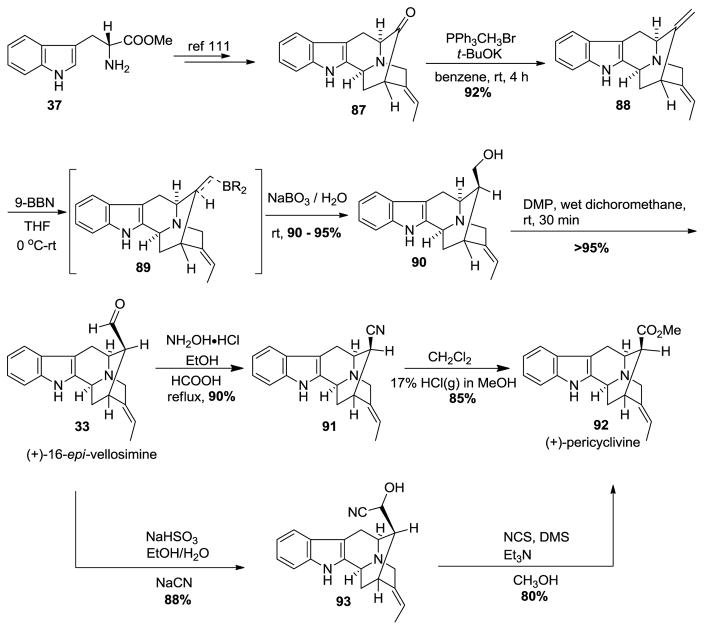
The synthesis of the northern vobasine fragment was accomplished from the previously synthesized (+)-16-epi-vellosimine (from D-(+)-tryptophan [110]). The E-ethylidene ketone 87 was available in an enantiospecific manner in five steps starting from commercially available D-(+)-tryptophan (Scheme 8) [111]. The Wittig olefination with methyltriphenylphosphonium bromide in the presence of potassium tert-butoxide in benzene provided the olefin 88 in excellent yield. Improved access to the aldehyde from the olefin via hydroboration with 9-BBN was followed by Kabalka oxidation with NaBO3. 4H2O to furnish (+)-16-epi-normacusine 90 in excellent yield. This was followed by Dess-Martin oxidation to yield the β-aldehyde, in (+)-16-epi-vellosimine 33 in >95% yield in the absence of any epimerization. The aldehyde was treated with hydroxylamine hydrochloride and subsequesnt dehydration of the so formed oxime which resulted in the corresponding cyano-compound 91 in 90% yield. The cyano function was converted into the corresponding methyl ester, (+)-pericyclivine 92, under the conditions (HCl(g), CH2Cl2, CH3OH) reported by Warmuth et al. [112]. In an alternative approach, (+)-16-epi-vellosimine 33 was converted into (+)-pericyclivine via a cyanohydrin that resulted from nucleophilic attack of the cyano group onto the bisulfite adduct of 16-epi-vellosimine analogous to a previous report by Wender et al. [113]. The cyanohydrin 93 was oxidized to the ketone at low temperature under Corey-Kim oxidation conditions, which yielded the ester (+)-pericyclivine 92 in 80% yield, on treatment with methanol. This constituted the first enantiospecific total synthesis of the indole alkaloid (+)-pericylivine.
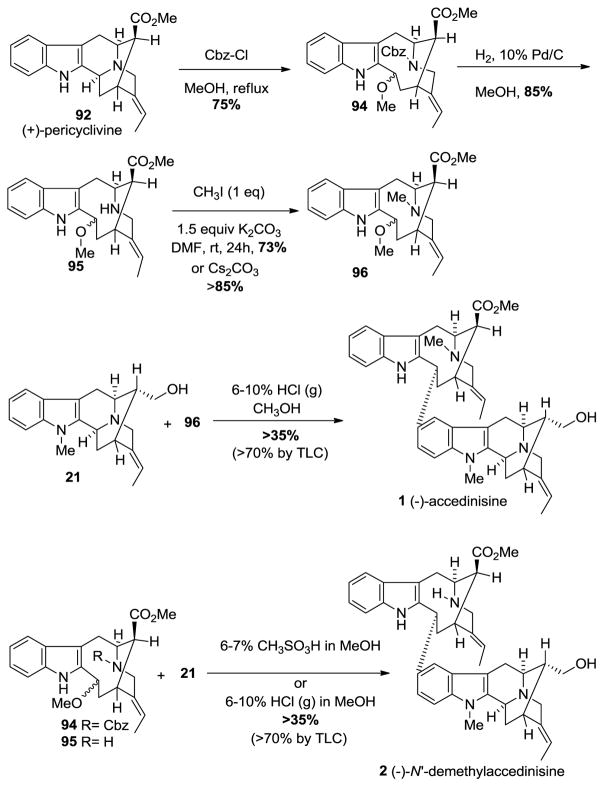
The C(3)-Nb bond cleavage [114–121] was carried out analogous to the procedure reported by Bonjoch et al. [114]. Pericyclivine 92 when heated to reflux in methanol, and reacted with benzyl chloroformate, to furnish the carbamate 94 in 75% yield (Scheme 9). The Cbz-group was removed by standard hydrogenolysis with hydrogen and palladium on carbon in methanol. This was followed by methylation of the Nb-group to provide the vobasine unit 96 required for (−)-accedinisine. The key to this synthesis was the formation of the 9-membered ring in 94 for the pseudo-axial ester and the pseudo-equatorial ester are similar in energy so there is no driving force for epimerization. This was in contrast to the β-ester function in 92, which can undergo epimerization to the α-configuration upon chromatography on silica gel.
Scheme 9.
Completion of the total synthesis of bisindole alkaloids (−)-1 and (−)-2.
(+)-Affinisine 21 is a sarpagine-type indole alkaloid isolated from Alstonia macrophylla [99] Wall, Peschiera affinis [122], and Ervatamia hirta [123]. In 2000, Liu et al. reported an enatiospecific total synthesis of the enatiomer of affnisine from L-(−)-tryptophan [124]. In 2006, Liao et al. completed the total synthesis of (+)-affinisine as an intermediate in the total synthesis of (+)-macroline and (−)-alstonerine [87].
The Nb-Cbz derivative 94 and Nb-methyl derivative 96 provided the northern hemispheres of (−)-N′-demethyl accedinisine 2 and (−)-accedinisine 1, respectively. The coupling of (+)-affininsine 21 with Nb-methyl intermediate 96 in 6%–7% methanolic HCl or with Nb-Cbz intermediate 94 in 6%–7% methanolic methanesulfonic acid or HCl(g) [125,126] provided the corresponding bisindoles in stereospecific fashion with an un-optimized yield of >35% (Scheme 9). There was one major component on TLC (silica gel) with a trace of a material too little to isolate with no starting materials left and no baseline material. Both of these dimers were run on milligram scale and were purified by preparative TLC on an actual thin layer plastic backed plate. The recovery was very poor. It is clear only one diastereomer was formed and from TLC the yield of the bisindoles appeared to be greater than 70%.
4.3. (+)-Macralstonine
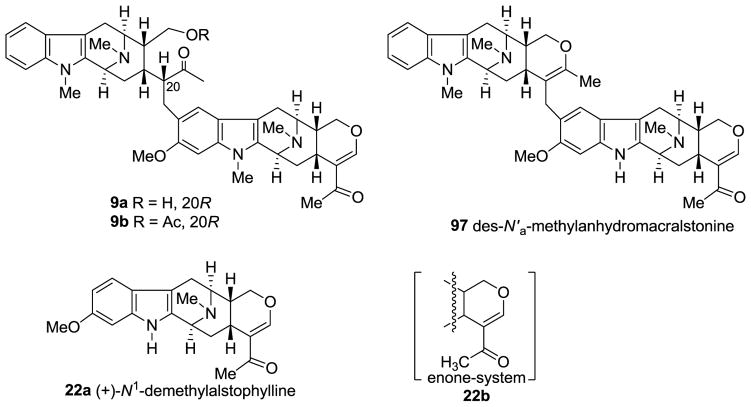
(+)-Macralstonine 9 is a dimeric alkaloid which consists of two macroline cores which belong to the Alstonia alkaloids (−)-alstophylline 22 and (+)-macroline 19. This bisindole was isolated from Alstonia macrophylla [22,127–130], Alstonia angustifolia [131], Alstonia muelleriana [132], and Alstonia glabriflora Mgf. [133]. Several related bisindoles (natural or semi-synthetic) with potent antimalarial activity, (+)-O-methylmacralstonine [22], (+)-O-acetylmacralstonine [40], and (+)-des-N′a-methylanhydromacralstonine 97 [73] were also known. In their studies, Schmid et al. [128] reported that (+)-macralstonine underwent ring opening and existed in an equilibrium mixture of the acyclic ketone 9a (Figure 7) and the cyclic hemiketal 9 in chloroform solution. This has been confirmed recently by Kam et al. by the analysis of high field NMR (600 MHz) spectroscopy that the ratio of acyclic to cyclic macralstonine was 2.32:1, 1.14:1, and 0:1 in CDCl3, CD2Cl2, and THF-d8 respectively. In their NMR (NOESY) spectroscopic studies of the O-methyl congener 11 during its isolation from A. macrophylla [22,130], Keawpradub et al. and Changwichit et al. concluded the stereochemistry at C-20 to be R (β-H). In the case of macralstonine [129], NOESY was not feasible. Fortunately, it was possible to trap the acyclic form as the O-acetyl derivative 9b (Figure 7) which clearly indicated the C-20 to be in R (β-H) configuration after spectroscopic analysis. This was further confirmed by X-ray analysis of (+)-macralstonine which was crystallized as the hemiketal 9 [129].
Figure 7.
Structures of the cyclic ketone form (9a) of (+)-macralstonine and its O-acetyl derivative (9b); des-N′a-methylanhydromacralstonine (97) as well N1-demethylalstophylline (22a), and the enone system (22b).
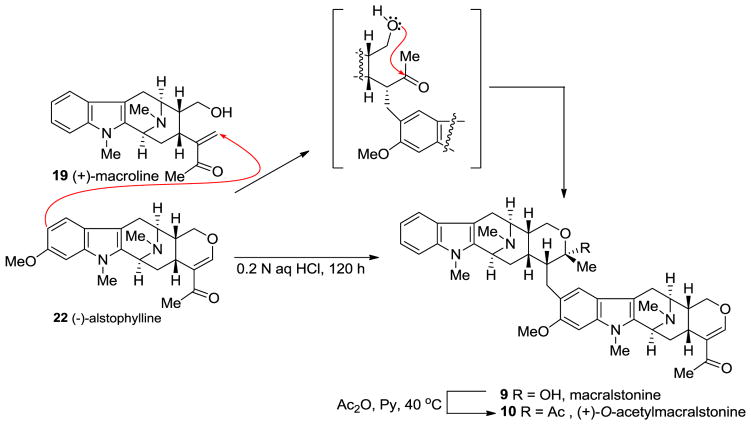
One possible biosynthetic route to (+)-macralstonine 9 was proposed by Schmid [134], which was confirmed in a biomimetic partial synthesis by LeQuesne et al. [135] by coupling (+)-macroline 19 with (−)-alstophylline 22 in dilute 0.2 N aqueous HCl solution (Scheme 10).
Scheme 10.
The biomimetic partial synthesis of macralstonine (9) and its O-acetyl derivative (10) by LeQuesne et al [135].
This 0.2 N aqueous hydrochloric acid solution which contained alstophylline and excess (+)-macroline was stirred at 20 °C for 5 days. (+)-Macralstonine 9 was isolated in 40% yield after chromatographic separation. Examination of the product by thin layer chromatography and infrared spectroscopy indicated this material was identical to the natural (+)-macralstonine and the analysis of the 1H NMR spectrum indicated the alkaloid was a mixture of C-20 epimers. Upon acetylation with actetic anhydride in pyridine, the C-20 epimer of macralstonine underwent epimerization to provide only (+)-O-acetylmacralstonine 10 indistinguishable from the acetyl derivative of natural (+)-macralstonine (Scheme 10) [40].
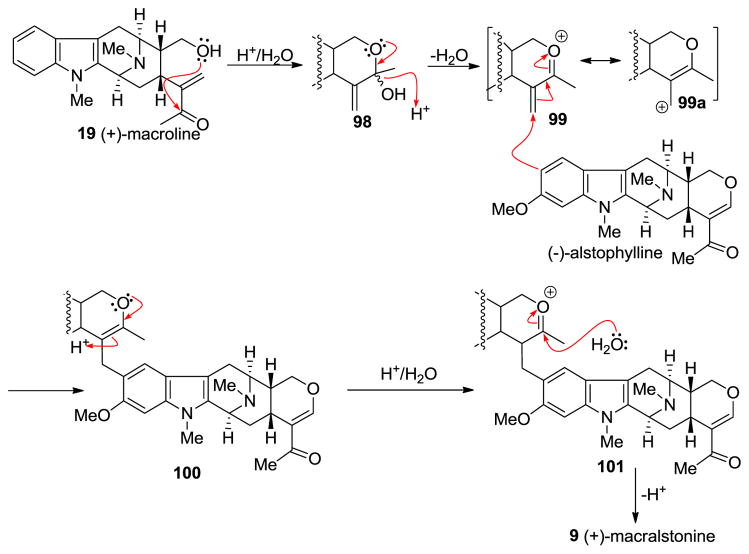
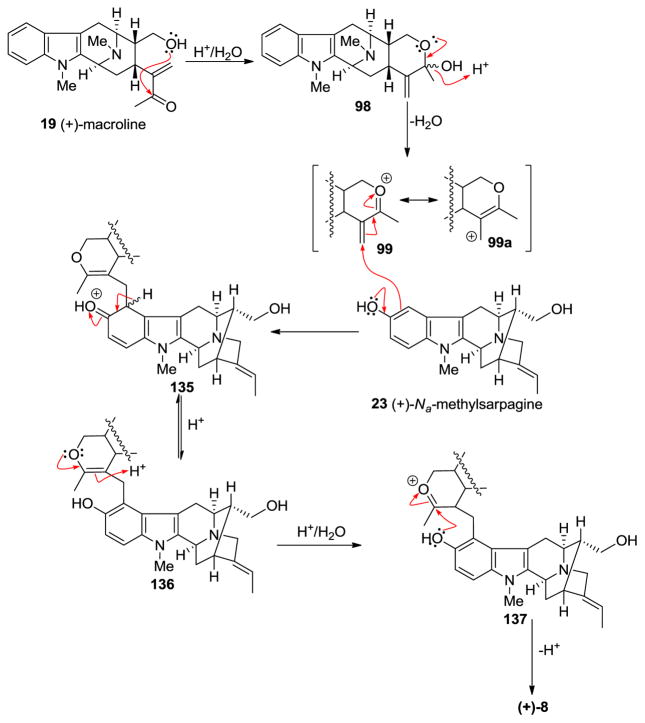
At least two mechanisms are possible for the formation of macralstonine 9 by the acid catalyzed coupling of macroline and the ring-A oxygenated macroline-type indole base (−)-alstophylline 22. One possible mechanism was the earlier described Michael process [135]; however another potential mechanism is a Friedel-Crafts alkylation process stabilized by the oxonium ion [136]. The Michael addition followed by cyclization is straight-forward, as shown in Scheme 10. The electron-rich aromatic ring adds nucleophiliclly in a 1,4-fashion to form the alkylated product ortho to the methoxy group, followed by nucleophilic addition of the C-17 hydroxy group to the carbonyl carbon atom to form the cyclic hemiketal present in macralstonine 9. On the other hand, alternatively, acid catalyzed intra-molecular cyclization of (+)-macroline, which involves the nucleophilic attack of the C-17 hydroxyl group on to the carbonyl carbon atom (1,2-fashion) would provide the cyclic hemiketal 98. This intermediate can then lose a molecule of water to form the oxonium ion species 99 (Figure 8). Electrophilic attack of the electron rich aromatic ring onto the oxonium ion electrophile would furnish the cyclic enol-ether 100, as suggested by Fukuyama. The cyclic enol-ether would then isomerize to an oxonium ion, only to be attacked by water to provide the cyclic hemiketal present in macralstonine 9.
Figure 8.
The possible alternative mechanism for the formation of (+)-9 via a Friedel-Crafts alkylation process.
(−)-Alstophylline 22, as described above comprises one unit of the antimalarial bisindoles macralstonine and the related O-acetyl and O-methyl macralstonine bases. This alkaloid was originally isolated from Alstonia macrophylla WALL by Kishi, Schmid et al. [78,128], from Alstonia glabriflora Mgf. by Hart [133] and from Alstonia angustifolia by Ghedira et al. [131]. In addition, the related Na-H analog (+)-N1-demethylalstophylline 22a has recently been isolated from Alstonia macrophylla by Kam [43].
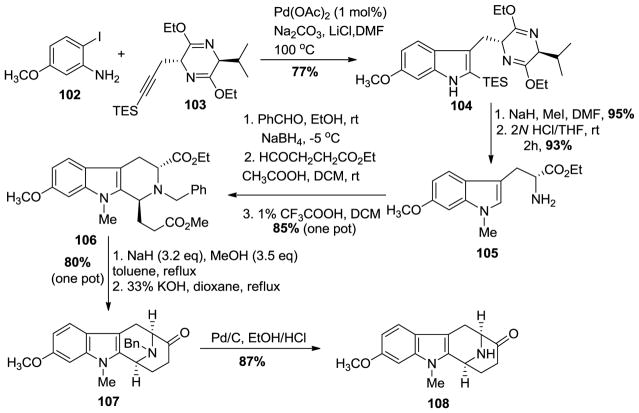
The total synthesis of (−)-alstophylline required facile access to the optically active 6-methoxy tryptophan 105 which was no easy task. It was developed by Huang, Wearing, Liu et al. [137,138] on large scale. In the initial approach towards several 11-methoxy macroline-related indole alkaloids, Liu et al. completed a total synthesis of (−)-alstophylline [139].
The readily available iodoaniline 102 and propargyl substituted Schöllkopf chiral auxiliary 103 [138] were coupled under Larock heteroannulation conditions regiospecifically to provide the 6-methoxyindole derivative 104 (Scheme 11). The Schöllkopf chiral auxiliary severved as a protecting group which prevented epimerization of the chiral center in strong base. The required Na-methylation was executed with sodium hydride and iodomethane in dimethyl formamide to furnish the Na-methylated 6-methoxytryptophan derivative, in greater than 98% ee. The hydrolysis of the dihydropyrazine moiety with aqueous 2N HCl in THF and concomitant removal of the triethylsilyl group resulted in the formation of Na-methyl-6-methoxy-D-(−)-tryptophan ethyl ester 105 in excellent yield. The primary amine 105 was converted into the benzyl protected amine by reduction of the phenyl substituted imine with sodium borohydride, which had formed by condensation of benzaldehyde with the primary amine. The asymmetric Pictet-Spengler reaction of the so formed Nb-benzyl protected tryptophan derivative with the aldehyde, ethyl 4-oxobutanoate under acidic conditions (HOAC in DCM) resulted in an almost quantitative yield of the di-ester 106 in a 72:28 (trans:cis) diastereomeric ratio. The same reaction in trifluoroacetic acid in DCM resulted in decomposition of the product. However, after the Pictet-Spengler reaction had been completed in acetic acid, the addition of a small amount of TFA was found to be effective for the epimerization of the diester at C-1 to the correscponding trans-diester isolated as a single diastereomer (>98% ee). The trans-diester 106 was subjected to a one-pot Dieckmann cyclization and this was followed by a base-mediated hydrolysis/decarboxylation process to provide the required 11-methoxy-Nb-benzyl protected tetracyclic ketone 107 in excellent yield. The catalytic hydrogenation on palladium on carbon under acidic conditions, removed the benzyl group to provide the desired the Nb-H tetracyclic ketone 108, as shown on Scheme 11.
Scheme 11.
Large scale enantiospecific access to the 11-methoxy substituted tetracyclic core structure present in (−)-alstophylline (22).
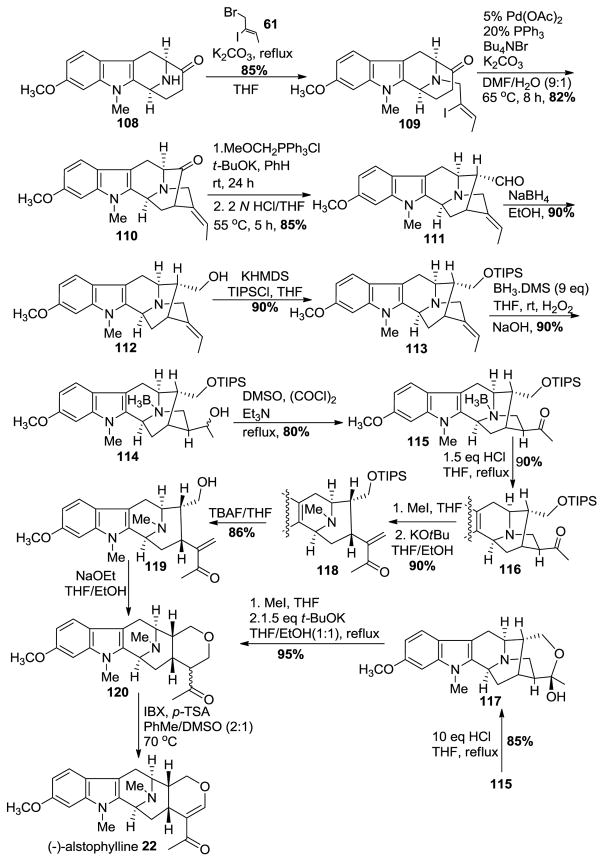
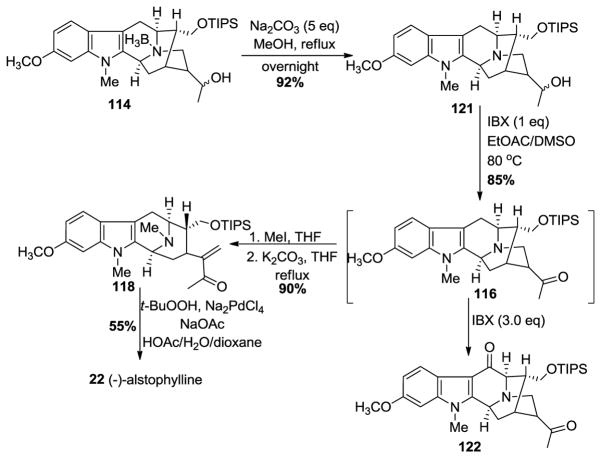
The Nb-H function in amine 108 was re-alkylated via an SN2 substitution with (Z)-1-bromo-2-iodo-2-butene 61 in the presence of K2CO3 in THF to provide the Nb-alkylated ketone 109 in good yield (Scheme 12) analogous to previous work. The iodo-2-butene 61 can now be prepared in 3 steps in stereospecific fashion on 500 gram scale. A palladium-catalyzed enolate-driven intramolecular cross-coupling process resulted in the pentacyclic ketone 110 in stereospecific fashion. A Wittig olefination was followed by hydrolysis of the enol ether which had resulted to yield the one-carbon homologated aldehyde 111 in excellent yield. The reduction of the aldehyde to the primary alcohol 112, to generate 11-methoxy affinisine was executed smoothly with sodium borohydride in ethanol. This constituted the first total synthesis of 112 (11-methoxy affinisine). Protection of the primary alcohol as a silyl ether 113 was done under standard conditions with triisopropylsilyl chloride in the presence of base. The hydroboration of the E-ethylidene function with borane-dimethyl sulfide and oxidation with basic hydrogen peroxide went smoothly to furnish the secondary alcohol 114, accompanied by a small amount of the corresponding tertiary alcohol. The Swern-oxidation conditions were employed to convert the mixture of secondary alcohols into the corresponding ketone 115. The Nb-BH3 adduct which had formed, could be converted into the free amine 116 by treating it with 1.5 equivalents of aqueous HCl in refluxing THF. On the other hand, in the presence of 10 equivalents of HCl in refluxing THF the cyclic hemiacetal 117 (11-methoxy-Na-methyl-trinervine) was isolated in 85% yield, which could be converted into 120 by quaternization of the Nb nitrogen atom with iodomethane. This was followed by treatment with base to provide a retro-Michael reaction which underwent recyclization to dihydroalstophylline 120.
Scheme 12.
Enantiospecific total synthesis of (−)-22 by Liu et al [139].
Alternatively, the ketone 116 underwent the ring opening process under conditions similar to LeQuesne et al. [140] to 120. The dihydroalstophylline upon treatment with IBX and p-TSA in toluene and DMSO gave (−)-alstophylline 22, albeit the yield of this step was only moderate.
In an improved approach, Liao et al. in Milwaukee developed a modified Wacker reaction to gain quick access to several macroline-type indole alkaloids (Scheme 13) [141]. The Nb-BH3 adduct 114 (Scheme 13) was accessed in similar fashion in better yield than the previous report by Liu et al. [139]. However, in this case, the Nb-BH3 group was removed alternatively by refluxing the complex with sodium carbonate in methanol to provide 121. The conditions of K.C. Nicolaou with IBX [142] were employed to oxidize the secondary alcohol to a ketone 116 (1 h) in 85% yield with the first equivalent of IBX. This was followed by addition of another 3 equivalents of IBX after the initial transformation and the mixture was heated to 80 °C. This promoted the radical process with IBX, as reported by Nicolaou et al. at the C-6 benzylic position to provide access to the 6-oxoalstophylline intermediate 122. This method permitted access to both alstophylline and its 6-oxo variant simply by changing the number of equivalents of IBX. The ring opening of the ketone 116 by a retro-Michael reaction under conditions modified from those of LeQuesne et al. gave the α,β-unsaturated system in 118. The ketone, under the modified Wacker conditions [143], effected the novel cyclization to provide (−)-alstophylline 22 directly. The total synthesis of alstophylline, accompanied by the total synthesis of (+)-macroline 19 by the group in Milwaukee constituted a total synthesis of (+)-macralstonine 9. In addition, the unique indole alkaloid 6-oxoalstophylline was synthesized for the first time, as well as a much improved route to (−)-alstonerine via this modified Wacker (Pd (II)) process. This process can be employed to prepare a number of enone systems (22b, Figure 7) found in indole alkaloids, as well as flavones (Lorenz, Cook et al.).
Scheme 13.
An improved synthesis of (−)-22 by Liao et al [141].
4.4. (+)-Macralstonidine
(+)-Macralstonidine 8 represents another dimeric indole alkaloid which consists of a macroline and a sarpagine obligate monomeric unit. It has been isolated from Alstonia macrophylla [127,128], Alstonia somersentenis [127], and Alstonia spectabilis [133]. The alkaloid, Na-methylsarpagine (23) is a natural product isolated from Alstonia spectabilis by Hart et al. [133]. Zhao et al. reported the first total synthesis of (+)-Na-methylsarpagine, as well as the total synthesis of (+)-macralstonidine, along with several other indole alkaloids [144,145]. The synthesis of (+)-Na-methylsarpagine accompanied by the total synthesis of (+)-macroline vide supra by the group in Milwaukee [85,86] constituted a total synthesis of this bisindole alkaloid.
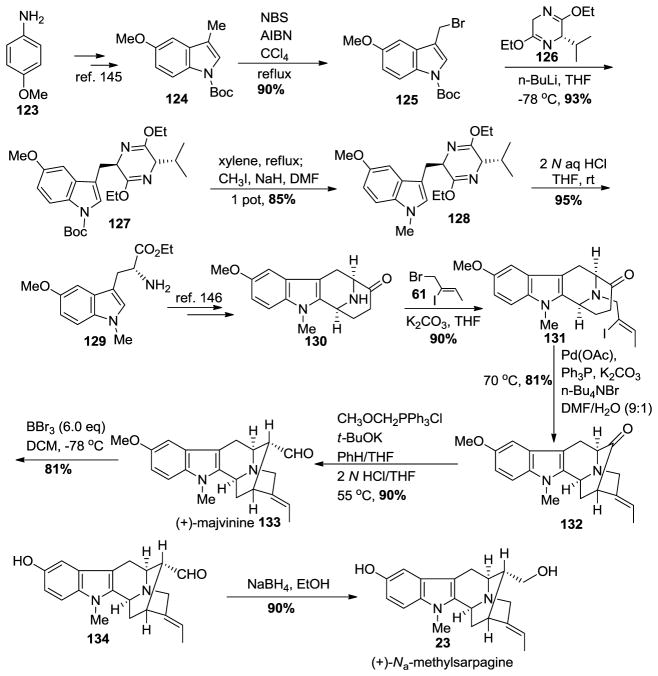
Synthesis of the desired sarpagine unit 23 began from p-anisidine 123 (Scheme 14) [145]. The 5-methoxy-3-methylindole-2-carboxylate was prepared on 600 gram scale via a Japp-Klingmann azo-ester intermediate [146]. The subsequent saponification was followed by a copper/quinoline-mediated decarboxylation process executed again on large scale in excellent yield. In this process only 2 equivalents of distilled quinoline were employed in contrast to the previous methods, which require a very large excess of quinoline. This latter route was difficult because the excess quinoline was hard to remove. The Na-H group was protected with a Boc function by treating it with Boc-anhydride and dimethyl amino pyridine to provide 124 in 95% yield. The regiospecific bromination of the C-3 alkyl group was done carefully under radical conditions with N-bromosuccinimide and AIBN (admixed and added in portions) in carbon tetrachloride on large scale and in excellent yield to furnish 125. This radical reaction was also executed in refluxing cyclohexane with similar results. The 3-bromomethyl indole was found to be very unstable and was used without further purification. It was treated with the anion of the Schöllkopf chiral auxiliary 126 at −78 °C to afford a single diastereomer 127 in 93% yield. The Boc group was removed under thermal conditions and this was followed by Na-methylation with iodomethane and sodium hydride in DMF to provide the Na-methyl tryptophan precursor 128. The dihydropyrazine moiety present in the Schöllkopf unit was hydrolyzed under acidic conditions in ethanol to furnish the 5-methoxy-Na-methyl D-(+)-tryptophan ethyl ester 129 on large scale. The usual subsequent transformations e.g., Nb-benzylation, asymmetric Pictet-Spengler condensation, diastereospecific Dieckmann cyclization-decarboxylation reaction and catalytic debenzylation on palladium/carbon to remove the Nb-benzyl group afforded the 5-methoxy tetracyclic ketone 130 in excellent overall yield. The Nb-nitrogen atom was re-alkylated with the vinyl iodide, (Z)-1-bromo-2-iodo-2-butene 61 (prepared regiospecifically on 500 gram scale) to give iodo olefin 131, which underwent the enolated-driven palladium-catalyzed cross-coupling to provide the α-vinylated intermediate, the key pentacyclic ketone 132 [144,145]. The conversion of the E-ethylidene intermediate 132 into the α-aldehyde [(+)-majvinine] 133 was effected using a one-carbon homologation sequence by Wittig olefination followed by hydrolysis/epimerization as employed previously in Milwaukee. The C-10 methoxy alkyl group was removed using 6.0 equivalents of boron tribromide in DCM at −78 °C to yield the 10-hydroxy intermediate 134 and this was followed by reduction of the aldehyde with sodium borohydride in ethanol to the primary alcohol in (+)-Na-methylsarpagine 23 in 90% yield over the two steps. This constituted the first enantiospecific total synthesis of (+)-majvinine and of (+)-Na-methylsarpagine 23 [144,145].
Scheme 14.
Enantiospecific total synthesis of (+)-23 by Zhao et al [144,145].
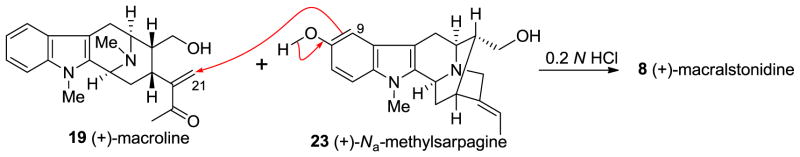
The condensation of (+)-macroline 19 and (+)-Na-methylsarpagine 23 under mildly acidicconditions provided the bisindole alkaloid, (+)-macralstonidine 8 (Scheme 15) [133]. In similar fashion to the case of macralstonine, there are at least two possible mechanisms of the coupling process. The first is the straight forward biomimetic coupling via a Michael reaction (Scheme 15) pioneered by LeQuesne [135], while the second is the Friedel-Crafts alkylation process [136,147], as illustrated in Figure 9. From a biological perspective the Michael reaction is the most reasonable, however, from a chemical perspective the Friedel-Crafts alkylation (Figure 9) is more likely. What is of significance here is that the alkylation took place soley at the C-9 position. No alkylation at C-11 was observed under these conditions.
Scheme 15.
Synthesis of (+)-8 by condensing (+)-macroline (19) and (+)-Na-methylsarpagine (23) under acidic conditions.
Figure 9.
The possible alternative mechanism for the formation of (+)-8 via a Friedel-Crafts alkylation process.
4.5. (+)-Villalstonine
Villalstonine 5 is a bisindole alkaloid consisting of two monomeric indole alkaloid units and Schmid et al. concluded from their study of the degradation of (+)-villalstonine that the monomeric components were (+)-macroline 19 and (+)-pleiocarpamine 14 [148]. Villalstonine has been isolated from the leaves and stem bark of Alstonia angustifolia [131,149], Alstonia macrophylla [22,128,148], Alstonia muelleriana [77], and Alstonia spectabilis [127]. The structure was deduced by Schmid et al. and confirmed by Nordman et al. by X-ray crystallography [150]. (+)-Pleiocarpamine, one unit, has been isolated from many Apocynaceae plants. To date, it has been isolated from Alstonia macrophylla [22], Alstonia angustifolia [131], Pleiocarpa mutica [151], Pleiocarpa pycnantha [152], Pleiocarpa tubicina [151], Kopsia dasyrachis [152], and from Hunteria eburnean Pichon [153] as well as Alstonia muelleriana [73]. The structure was based on examination of the NMR spectra, as well as in depth studies via mass spectrometry [154].
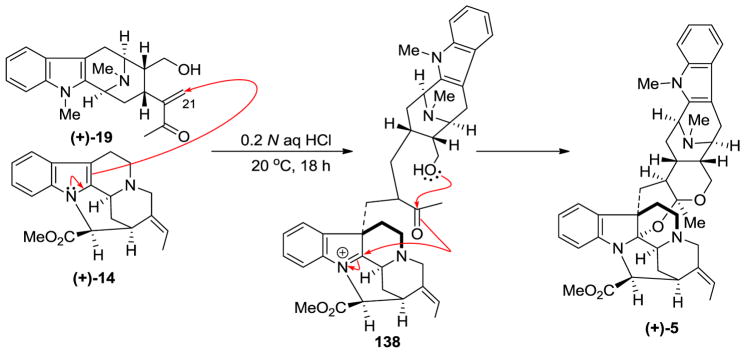
LeQuesne et al. executed a biomimetic synthesis of (+)-villalstonine 5 from condensation of (+)-macroline with (+)-pleiocarpamine 14 [83]. Macroline was obtained by degradation of villamine (not shown) and stirred with (+)-pleiocarpamine 14 in 0.2 N aqueous hydrochloric acid solution at 20 °C for 18 h. This was followed by basic workup to furnish (+)-villalstonine 5 indistinguishable from the natural product [83].
This biomimetic synthesis [83] of (+)-villalstonine is believed to consist of the initial electrophilic addition of the macroline enone 19 function onto pleiocarpamine 14 from the less hindered α-face to give intermediate 138. This intermediate undergoes facile transformation via hemiacetal formation and subsequent or concerted attack on the iminium ion of the indoline 138 to form the acetal-aminoactetal function in (+)-villalstonine 5, as illustrated in Scheme 16.
Scheme 16.
The biomimetic partial synthesis of (+)-5 by LeQuesne et al [83].
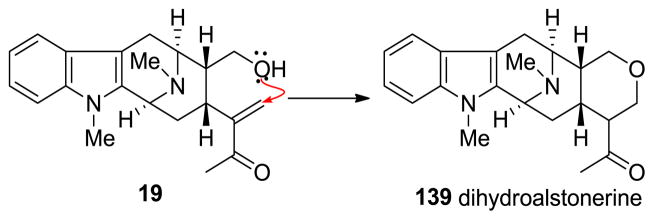
Since the total synthesis of (+)-pleiocarpamine 14 has not been successfully executed to date, the total synthesis of (+)-villalstonine 5 has still not been reported. In a partial synthesis of villalstonine, the Milwaukee group employed the more stable macroline equivalent 140, which served as a better alternative than macroline 19 itself, for the condensation with another monomeric unit to access bisindole alkaloids. This is because (+)-macroline tended to cyclize to form dihydroalstonerine 139 and limited the shelf life of macroline in storage (Scheme 17) [86].
Scheme 17.
Intramolecular cyclization of macroline (19) to form 139 which is responsible for its limited shelf life.
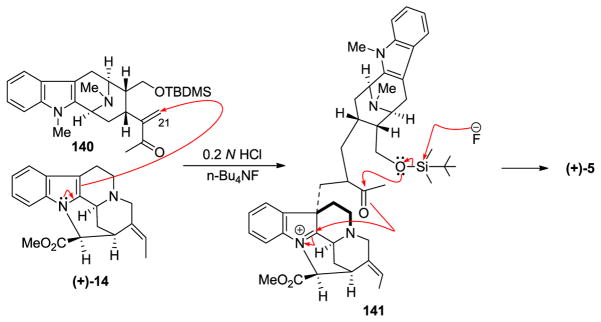
The stable macroline equivalent 140 was stirred with natural (+)-pleiocarpamine 14, obtained from Professor LeQuesne, in 0.2 N aqueous hydrochloric acid along with tetrabutylammonium fluoride at 20 °C for 24 h. (+)-Villalstonine 5 was obtained as the sole product and was indistinguishable from the natural (+)-villalstonine. It has not been confirmed whether the desilylation of the macroline equivalent 140 occurred to form macroline 19 before the condensation or the condensation took place similar to that depicted in Scheme 18. It is significant that the stable dihydroalstonerine 139 was not obtained in this process.
Scheme 18.
An improved partial synthesis of (+)-villalstonine (5) using the stable macroline equivalent (140) by Bi et al [86].
4.6. (−)-Macrocarpamine
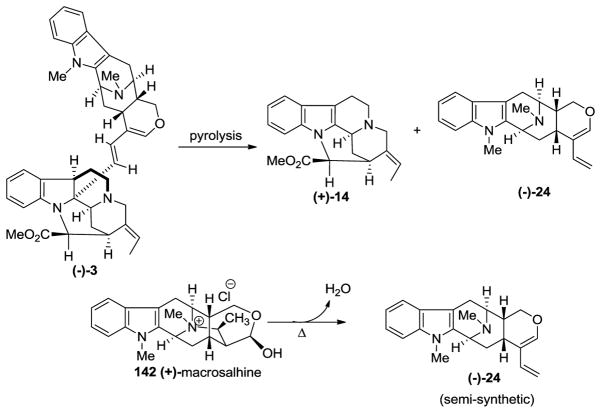
(−)-Macrocarpamine 3 is a bisindole arising from the condensation of (+)-pleiocarpamine 14 and the indole base (−)-anhydromacrosalhine-methine 24, according to the biogenetic pathway proposed by Hesse et al. [155]. It has been found in Alstonia macrophylla WALL [40,155], and Alstonia angustifolia [21,156]. (−)-Anhydromacrosalhine-methine 24 was first reported as a degradation product of (+)-macrosalhine 142 [157], as well as (−)-macrocarpamine 3 [155] (Scheme 19). The partial synthesis of macrocarpamine was carried out by Wang et al. from the condensation of natural (+)-pleiocarpamine 14 and semi-synthetic (see Scheme 19) (−)-anhydromacrosalhine-methine 24 [158–160].
Scheme 19.
Semi-synthetic access to (+)-14 and (−)-24 by the pyrolysis of (−)-macrocarpamine (3) and (+)-macrosalhine (142).
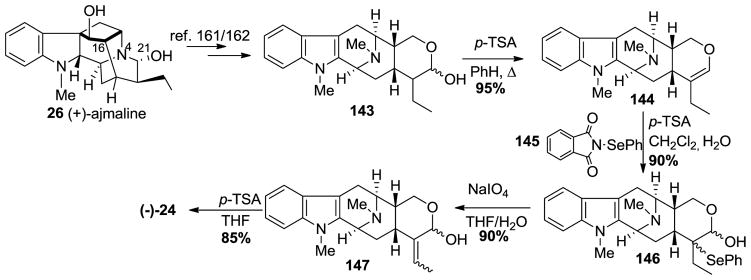
In the partial synthesis of (−)-macrocarpaine 3, an authentic sample of (−)-anhydromacrosalhine-methine 24 (a relay compound) was first prepared semi-synthetically from natural (+)-ajmaline 26 (Scheme 20) [160]. The hemiacetal 143 was prepared from ajmaline 26, according to the reported procedure by Sakai [161] and also carried out by Wearing et al. [162] which involved generation of the indole from the indoline, epimerization at C-16 and cleavage of Nb-C21 bond. This yielded the E ring in macrosalhine methine 24. In this sequence the hemiacetal 143 underwent a dehydration to form deoxyalstonerine 144 upon heating with p-toluenesulfonic acid in refluxing benzene. Oxyselenation of 144 to provide 146 was carried out using N-(phenylseleno)phthalimide in CH2Cl2 in the presence of 2–3 equivalents of water and 1.3 equivalents of p-toluenesulfonic acid. Elimination of the selenoxide upon treatment of 146 with NaIO4 provided the allylic alcohol 147. The 1,4-elimination in 147 of water was executed by stirring it with p-toluenesulfonic acid in THF to provide (−)-anhydromacrosalhine-methine 24 in 85% yield [160].
Scheme 20.
Semi-synthetic access to (−)-24 from relay compound (+)-ajmaline (26) [160].
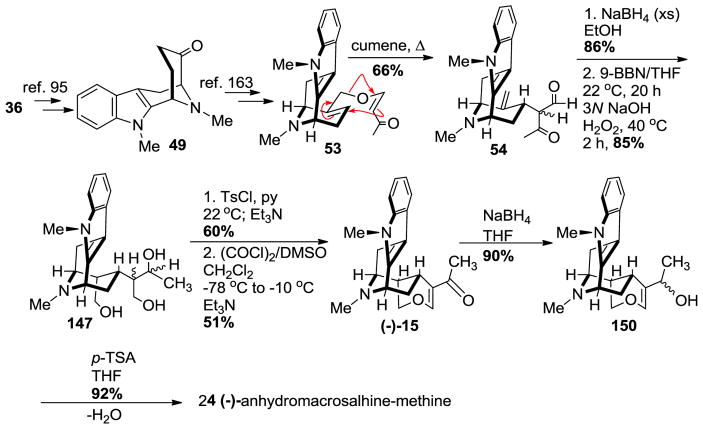
The enantiospecific total synthesis of (−)-anhydromacrosalhine-methine 24 began from D-(+)-tryptophan. The tetracyclic ketone 49 was prepared in >98% ee using the reported method (Scheme 21) [95] on large scale. (−)-Alstonerine 15 which was converted into (−)-24, was prepared from 49 following the same procedure reported for the total synthesis of alstonerine 15 by Bi et al. [86]. The enone ether 53 was synthesized from the Nb-methyl tetracyclic ketone 49 in four steps following the reported procedure [163] as described in brief here. The Claisen rearrangement of enone 53 took place from the desired α-face with at least 4:1 diastereoselectivity to provide 54 in 66% yield. Reduction of the carbonyl functions with sodium borohydride in ethanol was followed by stereospecific hydroboration-oxidation of the olefin with 9-BBN/THF and H2O2/NaOH which furnished the triol 147. The regioselective cyclization of 147 by using the tosylate as a leaving group generated the tetrahydropyran which was subsequently oxidized to the enone 15 [(−)-alstonerine] in 51% yield. The enone was accompanied by 31% of dihydroalstonerine 139 which could be transformed into the enone 15 through reduction and re-oxidation. Reduction of (−)-alstonerine 15 with sodium borohydride to the allylic alcohol 150 was followed by dehydration using p-TSA in excellent yield to complete the enantiospecific total synthesis of (−)-anhydromacrosalhine-methine 24.
Scheme 21.
Enantiospecific total synthesis of (−)-24 by Gan et al [158].
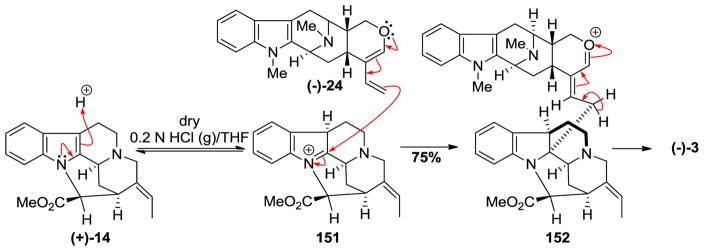
When (−)-anhydromacrosalhine-methine 24 was condensed under mildly aqueous acidic conditions (0.2 N aqueous HCl) with natural pleiocarpamine 14, the enol ether was converted into its hydrated form preventing it from undergoing the coupling reaction to yield (−)-macrocarpamine 3 [159]. However, under anhydrous conditions (0.2 N HCl (g)/THF), when 6 equivalents of 24 were added in portions over 48 h to (+)-pleiocarpamine, (−)-macrocarpamine 3 was formed in 75% yield (Scheme 22). A possible mechanism for the coupling reaction has been proposed by Wang et al. [159]. The proton acted as an electrophile to form the readily reversible iminium ion 151. The diene 24 was added portionwise in acidic solution to immediately quench the iminium ion upon its formation and to prevent the decomposition of the diene. The so formed (−)-macrocarpamine 3 was identical to the natural product reported by Hesse et al. by comparison of the spectral data [155].
Scheme 22.
Partial synthesis of (−)-macrocarpamine (3) by Gan et al [159].
4.7. (+)-Dispegatrine
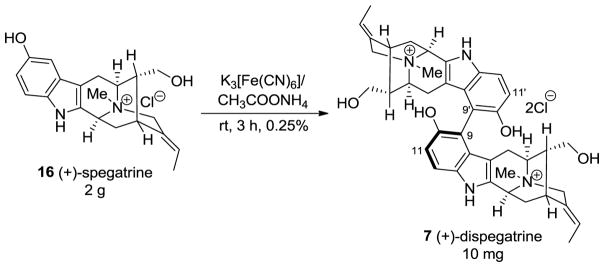
(+)-Dispegatrine 7 is a dimeric indole alkaloid isolated from the water-soluble fraction of the root extract of Rauwolfia verticillata (Lour.) Bail. var. hainanensis Tsiang [164,165]. It is a bisphenolic as well as a bisquaternary sarpagine indole alkaloid which makes it a unique alkaloid since the occurrence of these types of alkaloids are very rare [18]. (−)-Blumeanine (not shown) [166] is the only other alkaloid of this class among more than 300 or so bisindole alkaloids isolated to date. The monomer, spagatrine 16 is a major alkaloid isolated from Aspidosperma spegazzinii [167], R. sprucei [168], R verticillata var. hainanesis [164], and from R. verticillata var. rubrocarpa [165].
A biomimetic partial synthesis of (+)-dispegatrine 7 had been carried out from the monomer spegatrine 16 by Yu et al. [164] via an oxidative phenolic coupling in a negligible yield of 0.25% (Scheme 23). Although most of the structure of (+)-dispegatrine was determined based on the analysis of the NMR spectra and mass spectra, the partial synthesis by Yu confirmed the structure [164]. Moreover, no other diastereomer was reported. However, the isolation chemists were unable to determine the important axial chirality at the C9–C9′ bond [164]. In spite of that, formation of only one atropodiastereomer in that dehydrodimerization process suggested internal chiral induction was responsible for the atroposelectivity at the C9–C9′ bond. This strategy was employed in the total synthesis of (+)-dispegatrine 7 and its monomer, (+)-spegatrine 16 in a convergent fashion, by Edwankar et al. [169,170].
Scheme 23.
Biomimetic partial synthesis of dispegatrine by Yu et al. [164].
In a biomimetic total synthesis of the (P)-atropodiestereomer of (+)-dispegatrine [169,170], the tetracyclic ketone core 163 was accessed via the general process for ring-A oxygenated indole alkaloids, which began from 5-methoxy-D(+)-tryptophan via the asymmetric Pictet-Spengler condensation and diastereospecific Dieckmann cyclization process.
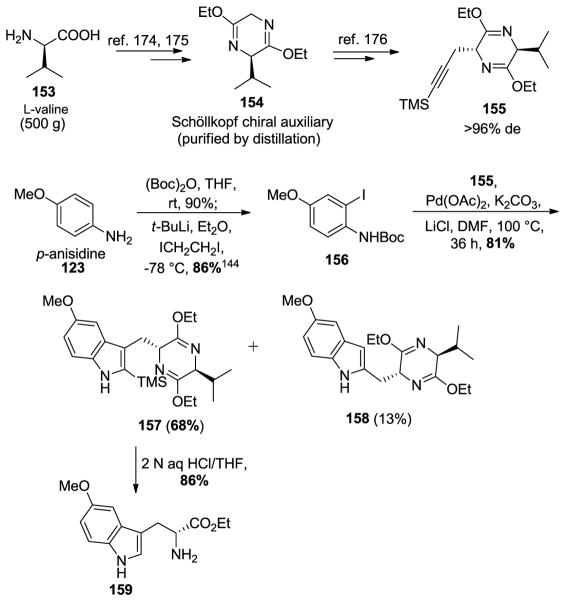
The enatiospecific synthesis of 5-methoxy tryptophan was done on large scale using a regiospecific bromination [145] and Larock heteroannulation [171–173] process. The L-valine in the Schöllkopf chiral auxiliary served both as the starting material and the source of chirality in the route. The Schöllkopf chiral auxiliary 154 was prepared on large scale (500 g) from L-valine 153 and glycine ethyl ester using an earlier method (Scheme 24) [174–176].
Scheme 24.
Enantiospecific access to 5-methoxy tryptophan (159) by The Larock heteroannulation process.
The o-iodoaniline 156 was synthesized by employing known methods (Scheme 24) [177,178]. The amino group in 123 was protected with a Boc group and subjected to a Snieckus ortho-lithiation [179] with 2.2 equivalents of tert-butyllithium at −78 °C and quenched with diiodoethane to afford the Boc protected iodoaniline 156 in 86% yield, required for the Larock heteroannulation. With the TMS-substituted alkyne 155 and iodoaniline 156 in hand, the heteroannulation was carried out using conditions developed by Ma et al. [176] which furnished the Schöllkopf analogs 157and 158 in a combined yield of 81%. Using a bulkier silyl TES-substituted alkyne did not improve the regioselectivity of the cyclization. Hydrolysis of indole 157 under acidic conditions removed the Schöllkopf unit, as well as the silyl group to provide optically active 5-methoxy-D-tryptophan ethyl ester 159 in 86% yield.
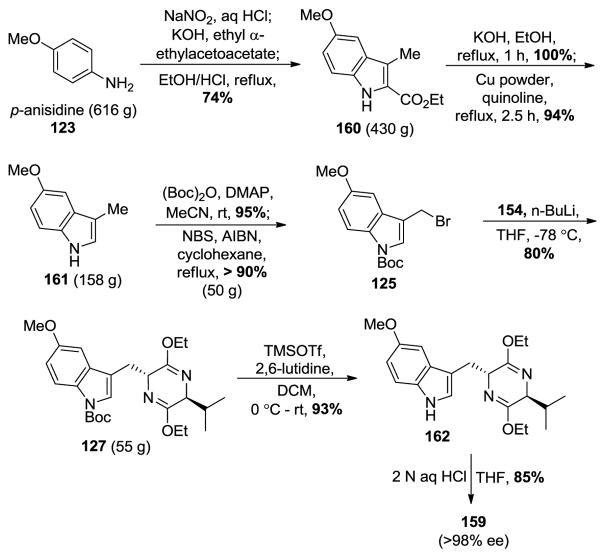
Due to the failure to improve the diastereoselectivity of the Larock heteroannulation (68:13) using the TMS-substituted alkyne 155 and the N-Boc protected ortho-iodo aniline 156 under the previously reported conditions [90,180], an alternative route involving a Japp-Klingemann/Fischer indole processes was also employed. This was developed by Abramovitch and Shapiro and was used here for the large-scale (>600 g) preparation of 3-methyl-5-methoxyindole 161 (Scheme 25) [181,182].
Scheme 25.
Alternative approach toward enantiopure 159 via the Japp-Klingemann/Fischer esterification process.
As mentioned above, the 5-methoxy-3-methylindole-2-carboxylate 160 was prepared on large scale via The Japp-Klingemann azo-ester intermediate (Scheme 25) [146]. The subsequent saponification was followed by a copper/quinoline-mediated decarboxylation executed on large scale to provide 161 in excellent yield. In order to carry out this decarboxylation in high yield only 2 equivalents of distilled quinoline were employed. The indole N-H group was then protected with a Boc group by treatment of 161 with Boc-anhydride and 4-(dimethylamino) pyridine in 95% yield. The subsequent regiospecific bromination of the C-3 alkyl group was done under radical conditions with N-bromosuccinimide and AIBN (admixed) in carbon tetrachloride on large scale in excellent yield to furnish bromide 125. This radical reaction could be carried out in refluxing cyclohexane with the same result. Alkylation of bromide 125 with the anion of the Schöllkopf chiral auxiliary (154) provided 127 on multigram scale. An improved and milder condition for the removal of the Boc group using TMSOTf/2,6-lutidine [183] was used in place of refluxing xylene [145]. The hydrolysis of the Schöllkopf analog 162 with 2 N aqueous HCl in THF provided the optically active Na-H, 5-methoxy-D-(−)-tryptophan ethyl ester (159) in excellent yield and diastereoselectivity (>98% ee).
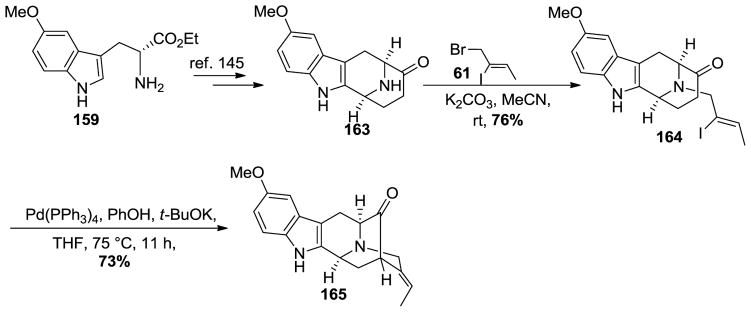
The tetracyclic ketone 163 was prepared on large scale from ester 159 which involved the asymmetric Pictet-Spengler reaction [37,145,184–186], and diastereospecific Dieckmann cyclization [111]. This was followed by base mediated decarboxylation and catalytic debenzylation of the Nb-benzyl function under acidic conditions (Scheme 26).
Scheme 26.
Synthesis of the 10-methoxy pentacyclic core system 165 from 159.
The Nb-alkylation was carried out by heating (Z)-1-bromo-2-iodo-2-butene 61 [187] with 163 in the presence of potassium carbonate in MeCN to provide the desired vinyl iodide 164 (Scheme 26) [111,145]. Because this route to 164 was somewhat longer, the Larock heteroannulation was often employed to prepare 5-methoxy-D-(+)-tryptophan. The enolate mediate palladium-catalyzed intramolecular cross-coupling of the vinyl iodide 164 was carried out under the conditions of Bonjoch [188] which employed PhOK/Pd(PPh3)4 to effect the coupling in 73% yield (Scheme 26). Initial attempts with the modified conditions (t-BuOK only) resulted in the pentacyclic ketone 165 in only 50% yield [87]. This was because the acetylene by product was formed in the presence of the stronger base t-BuOK versus the Bonjoch sodium phenoxide.
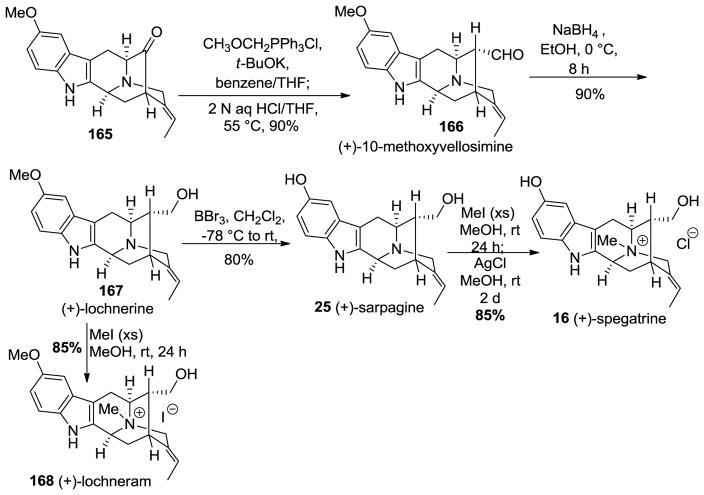
The E-ethylidene pentacyclic ketone 165 was subjected to a one-carbon homologation via a Wittig olefination-hydrolysis process employed many times in Milwaukee in a one pot sequence. This provided the desired α-aldehyde, the natural product (+)-10-methoxyvellosimine 166, in excellent yield (Scheme 27). The reduction of 166 with sodium borohydride yielded another natural product, (+)-lochnerine (167). Upon treatment with BBr3 in methylene chloride at −78 °C, demethylation of 167 occurred in 80% yield to furnish the hydroxyindole natural product (+)-sarpagine 25. The quaternization of 25 at the Nb-function using an excess of iodomethane was followed by simple iodide halide exchange with silver chloride in ethanol to furnish (+)-spegatrine chloride (16) [169,170]. In addition to (+)-lochnerine, when it was treated with an excess of iodomethane in methanol at rt for 24 h this provided the natural product (+)-lochneram 168 in 85% yield. These monomeric indole alkaloids were synthesized for the first time, the chemical and physical properties of which were very important in the final assault on (+)-dispegatrine. One could determine the chemical and chromatography characeteristics of these monomers to help with the isolation and purification of (+)-dispegatrine.
Scheme 27.
Enantiospecific total synthesis of (+)-10-methoxyvellosimine (166), (+)-lochnerine (167), (+)-sarpagine (25), (+)-spegatrine (16), and (+)-lochneram (168) by Edwankar et al [169,170].
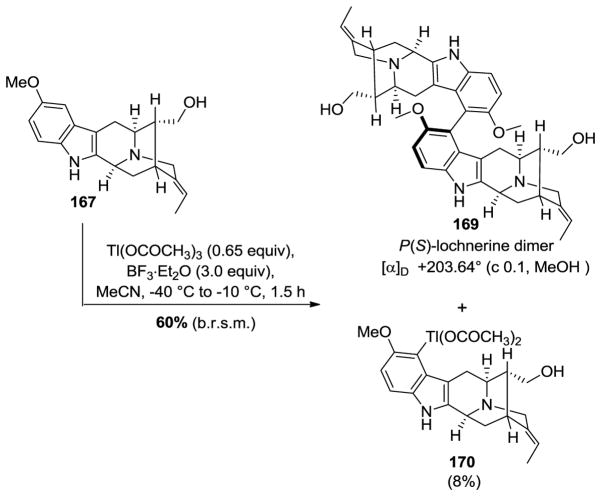
Numerous attempts were undertaken (including Ullmann coupling and Suzuki-Miyaura coupling) to effect the biaryl cross-coupling in model substrates while avoiding the very poor yields in the oxidative phenolic coupling reported in the partial synthesis of (+)-dispegatrine 7 by Yu et al. [170]. Eventually, a thallium(III) mediated Scholl-type dimerization [189] of (+)-lochnerine 167 was successfully executed wherein the chirality of the C9–C9′ bond was determined by the chirality inherent in the sarpagine system, as planned (Scheme 28).
Scheme 28.
Thallium (III) mediated oxidative dimerization process to provide P(S)-atropodiastereomer of the dimer lochnerine (169).
As shown in Scheme 28, The monomeric sarpagine alkaloid lochnerine 167, under the modified conditions of Taylor et al. [190,191] underwent Tl(III) mediated oxidative dimerization (a 0.65 equvalent of thallium (III) acetate with 3 equivalents of borontrifluoride etherate in MeCN at −40 °C) to furnish the key C9–C9′ biaryl coupling and provided the P(S)-atropodiastereomer 169 (confirmed by X-ray crystallographic analysis at low temperature wherein all the chiral centers were determined with the MoKα source) as the sole product and with complete regioselectivity [169]. This process was accompanied by a competing byproduct, an electrophilic thallation product 170 and its origin could be understood by the mechanistic studies of Kochi et al. [192,193].
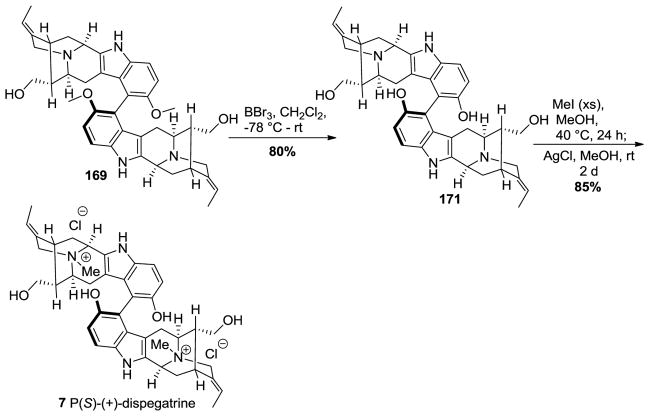
The methoxy group in 169 was demethylated using an excess of boron tribromide in methylene chloride at −78 °C to provide the sarpagine dimer 171 followed by Nb-quaternization using excess iodomethane in methanol at 40 °C to provide the dispegatrine diiodide salt (Scheme 29). Treatment of the diiodide salt with silver chloride in methanol provided (+)-dispegatrine 7 in 85% yield. Although the (P)-atropoisomer here could be correlated back to the configuration in the P(S)-(+)-lochnerine dimer, the stereochemistry was confirmed by examination of extensive NMR studies. This strategy completed the first total synthesis of the monomer spegatrine as well as the dimer P(S)-(+)-dispegatrine in a nature inspired and convergent manner in 8.3% yield starting from commercially available material. Moreover 6 other monomeric 10-methoxy substituted indole alkaloids were synthesized, enantiospecifically, for the first time.
Scheme 29.
Completion of the first total synthesis of P(S)-(+)-dispegatrine (7) by Edwankar et al [170].
5. Conclusions
Indole alkaloids of different classes and sub-classes have been a promising source of new drugs since the search for new molecular entities with profound biological activity from natural sources was initiated. It is sure to continue. The pace of natural product-based drug development from plants to patients has necessarily been rather moderate because of the difficulty in isolation of sufficient material for adequate structure determination, initial screening for a target, followed by preclinical and clinical studies. The anticancer properties of the important bisindole alkaloids, vincristine and vinblastine, prompted chemists including Potier, Kutney, Kuehne, Magnus, Rawal, and Fukuyama to execute elegant work in this area. In addition, natural product derivatives are an equally important area of further improvements in drug development. In the case of bisindoles, the bisindole itself continues to be more potent in most cases in comparison to the monomeric alkaloids which comprise them. It is this phenomenon that stimulated the interest in the synthesis of the antimalarial and antileishmanial bisindoles described herein. In order to accelerate the development of the drugs from natural sources, modern, practical, and efficient synthetic strategies capable of scale up to multigram quantities are of utmost importance. Illustrated in this review is the versatility of the asymmetric Pictet-Spengler reaction in the enantiospecific total synthesis of many biologically active monomeric and bisindole alkaloids. In addition, there are many other new developments with 100% diastereoselectivity derived from the coupling process of two monomeric alkaloids to provide the bisindole targets. Investigations of the synthesis of the monomeric unit has yielded many reactions capable of scale up, to date, to the 300–600 gram levels.
Acknowledgments
This work was supported by NS 076517 and MH 096463
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.O’Neil MJ. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. RSC Publishing; 2013. [Google Scholar]
- 2.Henry TA. The Plant Alkaloids. 4. J & A Churchill Ltd; London, UK: 1949. [Google Scholar]
- 3.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JF, Wei DQ, Chou KC. Drug candidates from traditional Chinese medicines. Curr Top Med Chem. 2008;8:1656–1665. doi: 10.2174/156802608786786633. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler MS. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 8.Cordell GA, Quinn-Beattie ML, Farnsworth NR. The potential of alkaloids in drug discovery. Phytother Res. 2001;15:183–205. doi: 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- 9.Tietze LF, Bell HP, Chandrasekhar S. Natural product hybrids as new leads for drug discovery. Angew Chem Int Ed. 2003;42:3996–4028. doi: 10.1002/anie.200200553. [DOI] [PubMed] [Google Scholar]
- 10.Mishra BB, Tiwari VK. Natural products: An evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 11.Cragg GM, Newman DJ. Biodiversity: A continuing source of novel drug leads. Pure Appl Chem. 2005;77:7–24. [Google Scholar]
- 12.Haefner B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 13.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 14.Butler MS. Natural products to drugs: Natural product derived compounds in clinical trials. Nat Prod Rep. 2005;22:162–195. doi: 10.1039/b402985m. [DOI] [PubMed] [Google Scholar]
- 15.Cragg GM, Newman DJ. Natural products: A continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordell GA, Saxton JE. Bisindole Alkaloids. In: Rodrigo GA, editor. The Alkaloids: Chemistry and Physiology. Vol. 20. Academic Press; New York, NY, USA: 1981. pp. 3–295. [Google Scholar]
- 17.Cordell GA. The bisindole alkaloids. In: Saxton JE, editor. Chemistry of Heterocyclic Compounds: Indoles, Part Four, the Monoterpenoid Indole Alkaloids. Vol. 25. John Wiley & Sons, Inc; New York, NY, USA: 1983. pp. 539–728. [Google Scholar]
- 18.Kam T-S, Choo Y-M. Bisindole alkaloids. In: Cordell GA, editor. The Alkaloids: Chemistry and Biology. Vol. 63. Academic Press; San Diego, CA, USA: 2006. pp. 181–337. [DOI] [PubMed] [Google Scholar]
- 19.Kitajima M, Takayama H. Chapter Four-Monoterpenoid Bisindole Alkaloids. In: Knölker H-J, editor. The Alkaloids: Chemistry and Biology. Vol. 76. Academic Press; San Diego, CA USA: 2016. pp. 259–310. [DOI] [PubMed] [Google Scholar]
- 20.Ban Y, Murakami Y, Iwasawa Y, Tsuchiya M, Takano N. Indole alkaloids in medicine. Med Res Rev. 1988;8:231–308. doi: 10.1002/med.2610080205. [DOI] [PubMed] [Google Scholar]
- 21.Wright C, Allen D, Cai Y, Phillipson J, Said I, Kirby G, Warhurst D. In vitro antiamoebic and antiplasmodial activities of alkaloids isolated from Alstonia angustifolia roots. Phytother Res. 1992;6:121–124. [Google Scholar]
- 22.Keawprdub N, Houghton P, Eno-Amooquaye E, Burke P. Activity of extracts and alkaloids of Thai Alstonia species against human lung cancer cell lines. Planta Med. 1997;63:97–101. doi: 10.1055/s-2006-957621. [DOI] [PubMed] [Google Scholar]
- 23.Johnson IS, Wright HF, Svoboda GH, Vlantis J. Antitumor principles derived from Vinca rosea Linn I. Vincaleukoblastine and leurosine. Cancer Res. 1960;20:1016–1022. [PubMed] [Google Scholar]
- 24.Noble R, Beer C, Cutts J. Role of chance observations in chemotherapy: Vinca rosea. Ann N Y Acad Sci. 1958;76:882–894. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]
- 25.Svoboda GH, Johnson IS, Gorman M, Neuss N. Current status of research on the alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don) J Pharm Sci. 1962;51:707–720. doi: 10.1002/jps.2600510802. [DOI] [PubMed] [Google Scholar]
- 26.Neuss N, Gorman M, Svoboda G, Maciak G, Beer C. Vinca alkaloids. III. 1. Characterization of leurosine and vincaleukoblastine, new alkaloids from Vinca rosea LINN. J Am Chem Soc. 1959;81:4754–4755. [Google Scholar]
- 27.Johnson I. Historical background of Vinca alkaloid research and areas of future interest. Cancer Chemother Rep. 1968;52:455–461. [PubMed] [Google Scholar]
- 28.Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from catharanthus. Alkaloids Chem Pharmacol. 1990;37:229–240. [Google Scholar]
- 29.Sieber S, Mead J, Adamson R. Pharmacology of antitumor agents from higher plants. Cancer Treat Rep. 1976;60:1127. [PubMed] [Google Scholar]
- 30.Carney DW, Lukesh JC, Brody DM, Brütsch MM, Boger DL. Ultrapotent vinblastines in which added molecular complexity further disrupts the target tubulin dimer–dimer interface. Proc Natl Acad Sci USA. 2016;113:9691–9698. doi: 10.1073/pnas.1611405113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutney JP. Synthetic Vinblastine and Vincristine Derivatives. 4144237 A. US Patent. 1979 Mar 13;
- 32.Fahy J, Duflos A, Ribet JP, Jacquesy JC, Berrier C, Jouannetaud MP, Zunino F. Vinca alkaloids in superacidic media: A method for creating a new family of antitumor derivatives. J Am Chem Soc. 1997;119:8576–8577. [Google Scholar]
- 33.Keglevich P, Hazai L, Kalaus G, Szántay C. Modifications on the basic skeletons of vinblastine and vincristine. Molecules. 2012;17:5893–5914. doi: 10.3390/molecules17055893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2005;5:65–71. doi: 10.2174/1568011053352569. [DOI] [PubMed] [Google Scholar]
- 35.Va P, Campbell EL, Robertson WM, Boger DL. Total synthesis and evaluation of a key series of C5-substituted vinblastine derivatives. J Am Chem Soc. 2010;132:8489–8495. doi: 10.1021/ja1027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J Am Chem Soc. 2009;131:4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenz M, Van Linn M, Cook J. The asymmetric Pictet-Spengler reaction. Curr Org Synth. 2010;7:189–223. [Google Scholar]
- 38.Munoz V, Moretti C, Sauvain M, Caron C, Porzel A, Massiot G, Richard B, Le Men-Olivier L. Isolation of bis-indole alkaloids with antileishmanial and antibacterial activities from Perschiera van heurkii (Syn. Tabernaemontana van heurkii) Planta Med. 1994;60:455–459. doi: 10.1055/s-2006-959531. [DOI] [PubMed] [Google Scholar]
- 39.Saxena S, Pant N, Jain D, Bhakuni R. Antimalarial agents from plant sources. Curr Sci. 2003;85:1314–1329. [Google Scholar]
- 40.Keawpradub N, Eno-Amooquaye E, Burke P, Houghton P. Cytotoxic activity of indole alkaloids from Alstonia macrophylla. Planta Med. 1999;65:311–315. doi: 10.1055/s-1999-13992. [DOI] [PubMed] [Google Scholar]
- 41.Wright CW, Allen D, Phillipson JD, Kirby GC, Warhurst DC, Massiot G, Le Men-Olivier L. Alstonia species: Are they effective in malaria treatment? J Ethnopharmacol. 1993;40:41–45. doi: 10.1016/0378-8741(93)90087-l. [DOI] [PubMed] [Google Scholar]
- 42.Wright CW, Phillipson JD. Natural products and the development of selective antiprotozoal drugs. Phytother Res. 1990;4:127–139. [Google Scholar]
- 43.Kam TS, Choo YM, Komiyama K. Unusual spirocyclic macroline alkaloids, nitrogenous derivatives, and a cytotoxic bisindole from Alstonia. Tetrahedron. 2004;60:3957–3966. [Google Scholar]
- 44.Feng Y, Gao H, Zeng G. Effect of higenamine on alpha-adrenoceptors. Acta pharmacol Sin. 1986;7:208–211. [PubMed] [Google Scholar]
- 45.Talapatra S, Aditya Chaudhury N. Macralstonine, an alkaloid of the trunk bark of Alstonia macrophylla Wall. Sci Cult. 1958;24:243. [Google Scholar]
- 46.Isidro N, Manalo GD. J Phillipine Pharm Assoc. 1967;53:8. [Google Scholar]
- 47.Keawpradub N, Kirby G, Steele J, Houghton P. Antiplasmodial activity of extracts and alkaloids of three Alstonia species from Thailand. Planta Med. 1999;65:690–694. doi: 10.1055/s-1999-14043. [DOI] [PubMed] [Google Scholar]
- 48.Kingston DG. Plant anticancer agents VI: Isolation of voacangine, voacamine, and epivoacorine from Tabernaemontana arborea sap. J Pharm Sci. 1978;67:271–272. doi: 10.1002/jps.2600670242. [DOI] [PubMed] [Google Scholar]
- 49.Kam TS, Tan SJ, Ng SW, Komiyama K. Bipleiophylline, an unprecedented cytotoxic bisindole alkaloid constituted from the bridging of two indole moieties by an aromatic spacer unit. Org Lett. 2008;10:3749–3752. doi: 10.1021/ol801354s. [DOI] [PubMed] [Google Scholar]
- 50.Koskinen A, Lounasmaa M. The sarpagine-ajmaline group of indole alkaloids. In: Ingham JL, editor. Progress in the Chemistry of Organic Natural Products. Vol. 43. Springer; Wien, Austria: 1983. pp. 267–346. [Google Scholar]
- 51.Brugada J, Brugada P. What to do in patients with no structural heart disease and sudden arrhythmic death? Am J Cardiol. 1996;78:69–75. doi: 10.1016/s0002-9149(96)00505-x. [DOI] [PubMed] [Google Scholar]
- 52.Creasey WA. Pharmacology, biochemistry, and clinical applications of the monoterpenoid alkaloids. In: Saxton JE, editor. Chemistry of Heterocyclic Compounds: Indoles, Part Four, The Monoterpenoid Indole Alkaloids. Vol. 25. John Wiley & Sons, Inc; New York, NY, USA: 1983. pp. 783–829. [Google Scholar]
- 53.Lounasmaa M, Hanhinen P, Westersund M, Halonen N. The Sarpagine Group of Indole Alkaloids. In: Cordell GA, editor. The Alkaloids: Chemistry and Biology. Vol. 52. Academic Press; San Diego, CA, USA: 1999. pp. 103–195. [Google Scholar]
- 54.Namjoshi OA, Cook JM. Chapter Two-Sarpagine and Related Alkaloids. In: Knölker H-J, editor. The Alkaloids: Chemistry and Biology. Vol. 76. Academic Press; San Diego, CA USA: 2016. pp. 63–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Men J, Taylor WI. A uniform numbering system for indole alkaloids. Experientia. 1965;21:508–510. doi: 10.1007/BF02138961. [DOI] [PubMed] [Google Scholar]
- 56.Hamaker LK, Cook JM. Chapter Two: The Synthesis of Macroline Related Sarpagine Alkaloids. In: Pelletier SW, editor. Alkaloids: Chemical and Biological Perspectives. Vol. 9. Elsevier Science; New York, NY, USA: 1995. pp. 23–84. [Google Scholar]
- 57.Esmond RW, Le Quesne PW. Biomimetic synthesis of macroline. J Am Chem Soc. 1980;102:7116–7117. [Google Scholar]
- 58.Woodward R. Neuere entwicklungen in der chemie der naturstoffe. Angew Chem. 1956;68:13–20. [Google Scholar]
- 59.Bartlett M, Lambert B, Werblood H, Taylor W. Rauwolfia alkaloids. XLIII. 1 A facile ring closure of deoxyajmalal-A to deoxyajmaline. J Am Chem Soc. 1963;85:475–477. [Google Scholar]
- 60.Van Tamelen E, Oliver L. The biogenetic-type total synthesis of ajmaline. Bioorg Chem. 1976;5:309–326. [Google Scholar]
- 61.Koskinen A, Lounasmaa M. Biogenesis of the Ajmaline Type Alkaloids. Planta Med. 1982;45:248–249. doi: 10.1055/s-2007-971385. [DOI] [PubMed] [Google Scholar]
- 62.Pfitzner A, Stöckigt J. Biogenetic link between sarpagine and ajmaline type alkaloids. Tetrahedron Lett. 1983;24:5197–5200. [Google Scholar]
- 63.Mattern-Dogru E, Ma X, Hartmann J, Decker H, Stöckigt J. Potential active-site residues in polyneuridine aldehyde esterase, a central enzyme of indole alkaloid biosynthesis, by modelling and site-directed mutagenesis. Eur J Biochem. 2002;269:2889–2896. doi: 10.1046/j.1432-1033.2002.02956.x. [DOI] [PubMed] [Google Scholar]
- 64.Ruppert M, Woll J, Giritch A, Genady E, Ma X, Stöckigt J. Functional expression of an ajmaline pathway-specific esterase from Rauvolfia in a novel plant-virus expression system. Planta. 2005;222:888–898. doi: 10.1007/s00425-005-0031-0. [DOI] [PubMed] [Google Scholar]
- 65.Yoneda N. Synthesis of 12-methyl-1,2,3,4,6,7,12,12b-octahydro-2, 6-methanoindolo[2,3-a]quinolizine. Chem Pharm Bull. 1965;13:622–625. doi: 10.1248/cpb.13.622. [DOI] [PubMed] [Google Scholar]
- 66.Mashimo K, Sato Y. On the synthesis of ajmaline. Tetrahedron Lett. 1969;10:905–906. [Google Scholar]
- 67.Cloudsdale IS, Kluge AF, McClure NL. Synthetic studies in the ajmaline series. J Org Chem. 1982;47:919–928. [Google Scholar]
- 68.Soerens D, Sandrin J, Ungemach F, Mokry P, Wu G, Yamanaka E, Hutchins L, DiPierro M, Cook J. Study of the Pictet-Spengler reaction in aprotic media: Synthesis of the. beta.-galactosidase inhibitor, pyridindolol. J Org Chem. 1979;44:535–545. [Google Scholar]
- 69.Zhang LH, Cook J. Pictet-Spengler reactions in aprotic media: Nb-benzyl promoted retention of optical activity in the synthesis of an indolo substituted azabicyclo[3.3. 1]nonane, a key template for the synthesis of macroline alkaloids. Heterocycles. 1988;27:2795–2802. [Google Scholar]
- 70.Li J, Wang T, Yu P, Peterson A, Weber R, Soerens D, Grubisha D, Bennett D, Cook J. General approach for the synthesis of ajmaline/sarpagine indole alkaloids: Enantiospecific total synthesis of (+)-ajmaline, alkaloid g, and norsuaveoline via the asymmetric pictet-spengler reaction. J Am Chem Soc. 1999;121:6998–7010. [Google Scholar]
- 71.Li J. PhD Thesis. University of Wisconsin-Milwaukee; Milwaukee, WI, USA: 1999. Enantiospecific Total Synthesis of (+)-ajmaline and Alkaloid G as Well as Studies Directed toward the Total Synthesis of 19-hydroxy-Nb-methylraumacline via the Asymmetric Pictet-Spengler Reaction. [Google Scholar]
- 72.Elderfield RC, Gilman RE. Alkaloids of Alstonia muelleriana. Phytochemistry. 1972;11:339–343. [Google Scholar]
- 73.Burke DE, Cook GA, Cook JM, Haller KG, Lazar HA, Le Quesne PW. Further alkaloids of Alstonia muelleriana. Phytochemistry. 1973;12:1467–1474. [Google Scholar]
- 74.Cook JM, Le Quesne P. Structure of alstonisidine, a novel dimeric indole alkaloid from Alstonia muelleriana. J Org Chem. 1971;36:582–586. [Google Scholar]
- 75.Burke DE, Cook JM, Le Quesne P. Biomimetic synthesis and structure of the bisindole alkaloid alstonisidine. J Chem Soc Chem Commun. 1972;697 doi: 10.1039/C39720000697. [DOI] [Google Scholar]
- 76.Hoard LG. PhD Thesis. University of Michigan; Ann Arbor, MI, USA: 1977. The Crystal Structures of Altstonisidine, C42H48N4O4, and Anhydrous Cholesterol, C27H46O. [Google Scholar]
- 77.Cook JM, Le Quesne P, Elderfield R. Alstonerine, a new indole alkaloid from Alstonia muelleriana. J Chem Soc Chem Commun. 1969:1306–1307. doi: 10.1039/C29690001306. [DOI] [Google Scholar]
- 78.Kishi T, Hesse M, Gemenden C, Taylor W, Schmid H. Alstophyllin, ein neues Indolalkaloid aus Alstonia macrophylla WALL. Helv Chim Acta. 1965;48:1349–1362. doi: 10.1002/hlca.19650480615. [DOI] [PubMed] [Google Scholar]
- 79.Aynilian G, Farnsworth N. Alkaloids of Vinca species. 3. Isolation and characterization of indole alkaloids from Vinca libanotica. Lloydia. 1974;37:299. [PubMed] [Google Scholar]
- 80.Salim AA, Garson MJ, Craik DJ. New indole alkaloids from the roots of Ochrosia a cuminata. J Nat Prod. 2004;67:1719–1721. doi: 10.1021/np049812p. [DOI] [PubMed] [Google Scholar]
- 81.Lyon R, Fong H, Farnsworth N, Svoboda G. Biological and phytochemical evaluation of plants XI: Isolation of aspidospermine, quebrachidine, rhazinilam, (−)-pyrifolidine, and akuammidine from Aspidosperma quebracho-blanco (apocynaceae) J Pharm Sci. 1973;62:218–221. doi: 10.1002/jps.2600620206. [DOI] [PubMed] [Google Scholar]
- 82.Gorman M, Burlingame A, Biemann K. Application of mass spectrometry to structure problems. The structure of quebrachidine. Tetrahedron Lett. 1963;4:39–46. [Google Scholar]
- 83.Burke DE, Cook JM, Le Quesne P. Biomimetic synthesis of the bisindole alkaloids villalstonine and alstonisidine. J Am Chem Soc. 1973;95:546–552. [Google Scholar]
- 84.Hesse M, Bodmer F, Gemenden C, Joshi B, Taylor W, Schmid H. Die struktur des Alstonia-alkaloides villalstonin. Helv Chim Acta. 1966;49:1173–1182. doi: 10.1002/hlca.19650480405. [DOI] [PubMed] [Google Scholar]
- 85.Bi Y, Cook JM. General approach for the synthesis of macroline/sarpagine alkaloids. The total synthesis of (+)-macroline. Tetrahedron Lett. 1993;34:4501–4504. [Google Scholar]
- 86.Bi Y, Zhang LH, Hamaker LK, Cook JM. Enantiospecific Synthesis of (−)-Alstonerine and (+)-Macroline as Well as a Partial Synthesis of (+)-Villalstonine. J Am Chem Soc. 1994;116:9027–9041. [Google Scholar]
- 87.Liao X, Zhou H, Yu J, Cook JM. An improved total synthesis of (+)-macroline and alstonerine as well as the formal total synthesis of (−)-talcarpine and (−)-anhydromacrosalhine-methine. J Org Chem. 2006;71:8884–8890. doi: 10.1021/jo061652u. [DOI] [PubMed] [Google Scholar]
- 88.Yu J, Wang T, Liu X, Deschamps J, Flippen-Anderson J, Liao X, Cook JM. General approach for the synthesis of sarpagine indole alkaloids. Enantiospecific total synthesis of (+)-vellosimine, (+)-normacusine B, (−)-alkaloid Q3, (−)-panarine, (+)-Na-methylvellosimine, and (+)-Na-methyl-16-epi-pericyclivine. J Org Chem. 2003;68:7565–7581. doi: 10.1021/jo030006h. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Zhang C, Liao X, Cook JM. Enantiospecific total synthesis of the enantiomer of the indole alkaloid intermediate macroline. Tetrahedron Lett. 2002;43:7373–7377. [Google Scholar]
- 90.Yu J, Wearing XZ, Cook JM. A general strategy for the synthesis of vincamajine-related indole alkaloids: Stereocontrolled total synthesis of (+)-dehydrovoachalotine, (−)-vincamajinine, and (−)-11-methoxy-17-epivincamajine as well as the related quebrachidine diol, vincamajine diol, and vincarinol. J Org Chem. 2005;70:3963–3979. doi: 10.1021/jo040282b. [DOI] [PubMed] [Google Scholar]
- 91.Neukomm G, Kletzhäundler E, Hesse M. Die absolute konfiguration von macrolin, einem abbauprodukt des alkaloides villalstonin 179. Mitteilung über organische naturstoffe. Helv Chim Acta. 1981;64:90–96. [Google Scholar]
- 92.Gorman M, Sweeny J. Perivine. Tetrahedron Lett. 1964;5:3105–3111. [Google Scholar]
- 93.Zhang LH, Cook JM. Pictet-Spengler reactions in aprotic media. Stereospecific conversion of optically active cis-1, 3-disubstituted 1,2,3,4-tetrahydro-β-carbolines into their corresponding trans diastereomers. Heterocycles. 1988;27:1357–1363. [Google Scholar]
- 94.Yu P, Wang T, Yu F, Cook JM. General approach for the synthesis of macroline/sarpagine related indole alkaloids via the asymmetric pictet-spengler reaction: the enantiospecific synthesis of the Na-H, azabicyclo[3.3.1]nonone template. Tetrahedron Lett. 1997;38:6819–6822. [Google Scholar]
- 95.Zhang LH, Bi YZ, Yu FX, Menzia G, Cook JM. Stereospecificity in the Pictet-Spengler reaction. Enantiospecific synthesis of (6S, 10S)-5#75)-5-methyl-9-oxo-12-benzyl-6,7,8,9,10,11-hexahydro-6,10-imino-5H-cyclooct[b]indole, a template for preparation of macroline/sarpagine alkaloids. Heterocycles. 1992;34:517–547. [Google Scholar]
- 96.Trudell ML, Cook JM. Total synthesis of (±)-suaveoline. J Am Chem Soc. 1989;111:7504–7507. [Google Scholar]
- 97.Bornack WK, Bhagwat SS, Ponton J, Helquist P. Preparation of lactone systems. Total synthesis of (±)-quadrone. J Am Chem Soc. 1981;103:4647–4648. [Google Scholar]
- 98.Amer MA, Court WE. Alkaloids of Rauwolfia nitida root bark. Phytochemistry. 1981;20:2569–2573. [Google Scholar]
- 99.Banerji A, Chakrabarty M, Mukherjee B. Minor indole alkaloids of Alstonia macrophylla. Phytochemistry. 1972;11:2605–2607. [Google Scholar]
- 100.Tran YS, Kwon O. An application of the phosphine-catalyzed [4 + 2] annulation in indole alkaloid synthesis: Formal syntheses of (±)-alstonerine and (±)-macroline. Org Lett. 2005;7:4289–4291. doi: 10.1021/ol051799s. [DOI] [PubMed] [Google Scholar]
- 101.Fukuyama T, Cheung M, Kan T. N-carboalkoxy-2-nitrobenzenesulfonamides: A practical preparation of N-Boc-, N-Alloc-, and N-Cbz-protected primary amines. Synlett. 1999;1999:1301–1303. [Google Scholar]
- 102.Eschweiler W. Ersatz von an stickstoff gebundenen wasserstoffatomen durch die methylgruppe mit hülfe von formaldehyd. Ber Dtsch Chem Ges. 1905;38:880–882. [Google Scholar]
- 103.Clarke H, Gillespie H, Weisshaus S. The action of formaldehyde on amines and amino acids1. J Am Chem Soc. 1933;55:4571–4587. [Google Scholar]
- 104.Achenbach H, Schaller E. Alkaloide in Tabernaemontana-Arten, VII. Über einige Bisindolalkaloide aus Tabernaemontana accedens. Chem Ber. 1976;109:3527–3536. [Google Scholar]
- 105.Azoug M, Loukaci A, Richard B, Nuzillard JM, Moreti C, Zèches-Hanrot M, Le Men-Olivier L. Alkaloids from stem bark and leaves of Peschiera buchtieni. Phytochemistry. 1995;39:1223–1228. [Google Scholar]
- 106.Yang J, Sarma P, Cook JM. Progress toward the enantiospecific total synthesis of accedinisine and N′-demethylaccedinisine. Proceedings of the 235th American Chemical Society National Meeting; New Orleans, LA, USA. 6–10 April 2008; ORGN 648. [Google Scholar]
- 107.Rallapalli SK, Cook JM. Progress toward the total synthesis of the sarpagine related alkaloids amervolfine and ervincidine. Proceedings of the 237th American Chemical Society National Meeting; Salt Lake City, UT, USA. 22–26 March; 2009; ORGN 517. [Google Scholar]
- 108.Edwankar CR, Edwankar RV, Namjoshi OA, Rallapalli SK, Yang J, Cook JM. Recent progress in the total synthesis of indole alkaloids. Curr Opin Drug Discov Devel. 2009;12:752–771. [PubMed] [Google Scholar]
- 109.Edwankar CR, Edwankar RV, Rallapalli S, Cook JM. General approach to the total synthesis of macroline-related sarpagine and ajmaline alkaloids. Nat Prod Commun. 2008;3:1839–1870. [Google Scholar]
- 110.Yin W, Ma J, Rivas FM, Cook JM. First enantiospecific total synthesis of the important biogenetic intermediates, (+)-polyneuridine and (+)-polyneuridine aldehyde, as well as 16-epi-vellosimine and macusine A. Org Lett. 2007;9:295–298. doi: 10.1021/ol062762q. [DOI] [PubMed] [Google Scholar]
- 111.Wang T, Cook JM. General approach for the synthesis of sarpagine/ajmaline indole alkaloids. stereospecific total synthesis of the sarpagine alkaloid (+)-vellosimine. Org Lett. 2000;2:2057–2059. doi: 10.1021/ol000095+. [DOI] [PubMed] [Google Scholar]
- 112.Warmuth R, Munsch TE, Stalker RA, Li B, Beatty A. Enantioselective synthesis of benzocyclic α, α-dialkyl-amino acids: New insight into the solvent dependent stereoselectivity of the TMSCN addition to phenylglycinol derived imines. Tetrahedron. 2001;57:6383–6397. [Google Scholar]
- 113.Wender PA, Schaus JM, White AW. General methodology for cis-hydroisoquinoline synthesis: Synthesis of reserpine. J Am Chem Soc. 1980;102:6157–6159. [Google Scholar]
- 114.Bonjoch J, Fernàndez JC, Valls N. Total syntheses of (±)-deethylibophyllidine using a crisscross annulation: Ring cleavage of octahydroindolo[2,3-a]quinolizines followed by tandem cyclizations of octahydroazecino[5,4-b]indoles. J Org Chem. 1998;63:7338–7347. doi: 10.1021/jo980909o. [DOI] [PubMed] [Google Scholar]
- 115.Schill G, Löwer H, Priester CU, Windhövel UF, Fritz H. Eine neue synthese von vinblastin-derivaten II: Synthesekonzept und modelluntersuchungen. Tetrahedron. 1987;43:3729–3745. [Google Scholar]
- 116.Schill G, Priester CU, Windhovel UF, Fritz H. Eine neue synthese von vinblastin-derivaten v. konzept und untersuchungen zur synthese von 20′-desethyl-20′-desoxy-c′-homovinblastin—octahydro-3H-azecino[5,4-b]inidol-derivate. Tetrahedron. 1990;46:1211–1220. [Google Scholar]
- 117.Calverley M, Harley-Mason J, Quarrie S, Edwards P. On the stereochemistry of the solvolytic c/d ring cleavage of the 1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizine system. Tetrahedron. 1981;37:1547–1556. [Google Scholar]
- 118.Liu CT, Sun SC, Yu QS. Synthesis and photooxidation of the condensation products of tryptamine and catechol derivatives. An approach to the synthesis of a probable precursor of koumine. J Org Chem. 1983;48:44–47. [Google Scholar]
- 119.Takayama H, Masubuchi K, Kitajima M, Aimi N, Sakai SI. A biomimetic construction of humantenine skeleton. Tetrahedron. 1989;45:1327–1336. [Google Scholar]
- 120.Takayama H, Tominaga Y, Kitajima M, Aimi N, Sakai SI. First synthesis of the novel Gelsemium alkaloids, gelselegine, gelsenicine, and gelsedine using a biomimetic approach. J Org Chem. 1994;59:4381–4385. [Google Scholar]
- 121.Banks B, Calverley M, Edwards P, Harley-Mason J. A new synthesis of indolo[2,3-α]quinolizidine derivatives: A formal total synthesis of (±)-geissoschizine. Tetrahedron Lett. 1981;22:1631–1634. [Google Scholar]
- 122.Weisbach JA, Raffauf RF, Ribeiro O, Macko E, Douglas B. Problems in chemotaxonomy I. Alkaloids of Peschiera affinis. J Pharm Sci. 1963;52:350–353. doi: 10.1002/jps.2600520408. [DOI] [PubMed] [Google Scholar]
- 123.Clivio P, Richard B, Deverre JR, Sevenet T, Zeches M, Le Men-Oliver L. Alkaloids from leaves and root bark ofErvatamia hirta. Phytochemistry. 1991;30:3785–3792. [Google Scholar]
- 124.Liu X, Wang T, Xu Q, Ma C, Cook JM. Enantiospecific total synthesis of the enantiomer of the indole alkaloid affinisine. Tetrahedron Lett. 2000;41:6299–6303. [Google Scholar]
- 125.Buchi G, Manning R, Monti S. Voacamine and voacorine. J Am Chem Soc. 1964;86:4631–4641. [Google Scholar]
- 126.Buchi G, Manning R, Monti S. Voacamine. J Am Chem Soc. 1963;85:1893–1894. [Google Scholar]
- 127.Sharp TM. 265. The alkaloids of Alstonia barks. Part II. A. macrophylla, wall., A. somersetensis, FM Bailey, A. verticillosa, F. Muell., A. villosa, blum. J Chem Soc. 1934:1227–1232. doi: 10.1039/JR9340001227. [DOI]
- 128.Kishi T, Hesse M, Vetter W, Gemenden C, Taylor W, Schmid H. Macralstonin. Helv Chim Acta. 1966;49:946–964. [Google Scholar]
- 129.Lim SH, Low YY, Tan SJ, Lim KH, Thomas NF, Kam TS. Perhentidines A–C: Macroline-macroline bisindoles from Alstonia and the absolute configuration of perhentinine and macralstonine. J Nat Prod. 2012;75:942–950. doi: 10.1021/np300120p. [DOI] [PubMed] [Google Scholar]
- 130.Changwichit K, Khorana N, Suwanborirux K, Waranuch N, Limpeanchob N, Wisuitiprot W, Suphrom N, Ingkaninan K. Bisindole alkaloids and secoiridoids from Alstonia macrophylla Wall.ex G. Don. Fitoterapia. 2011;82:798–804. doi: 10.1016/j.fitote.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 131.Ghedira K, Zeches-Hanrot M, Richard B, Massiot G, Le Men-Olivier L, Sevenet T, Goh S. Alkaloids of Alstonia angustifolia. Phytochemistry. 1988;27:3955–3962. [Google Scholar]
- 132.Cook J, Le Quesne P. Macralstonine from Alstonia muelleriana. Phytochemistry. 1971;10:437–439. [Google Scholar]
- 133.Hart N, Johns S, Lamberton J. Tertiary alkaloids of Alstonia spectabilis and Alstonia glabriflora (Apocynaceae) Aust J Chem. 1972;25:2739–2741. [Google Scholar]
- 134.Schmid H. 4 Internationales Symposium Biochemie und Physiologie der Alkaloide (1969) Akademie Verlag; Berlin, Germany: 1972. p. 348. [Google Scholar]
- 135.Burke DE, DeMarkey CA, Le Quesne P, Cook JM. Biomimetic synthesis of the bis-indole alkaloid macralstonine. J Chem Soc Chem Commun. 1972:1346–1347. doi: 10.1039/C39720001346. [DOI] [Google Scholar]
- 136.Liu X. PhD Thesis. University of Wisconsin-Milwaukee; Milwaukee, WI, USA: 2002. The Enantiospecific Stereospecific Total Synthesis of the Enantiomers of the Indole Alkaloids Na-methylvellosimine, Affinisine and Macroline as Well as the Total Synthesis of the Indole Alkaloids Trinervine, Alstophylline and the Antimalarial Bisindole Macralstonine. [Google Scholar]
- 137.Allen MS, Hamaker LK, La Loggia AJ, Cook JM. Entry into 6-methoxy-D(+)-tryptophans. Stereospecific synthesis of 1-benzenesulfonyl-6-methoxy-D(+)-tryptophan ethyl ester. Synth Commun. 1992;22:2077–2102. [Google Scholar]
- 138.Ma C, Liu X, Li X, Flippen-Anderson J, Yu S, Cook JM. Efficient asymmetric synthesis of biologically important tryptophan analogues via a palladium-mediated heteroannulation reaction. J Org Chem. 2001;66:4525–4542. doi: 10.1021/jo001679s. [DOI] [PubMed] [Google Scholar]
- 139.Liu X, Deschamp JR, Cook JM. Regiospecific, enantiospecific total synthesis of the alkoxy-substituted indole bases, 16-epi-Na-methylgardneral, 11-methoxyaffinisine, and 11-methoxymacroline as well as the indole alkaloids alstophylline and macralstonine. Org Lett. 2002;4:3339–3342. doi: 10.1021/ol020101x. [DOI] [PubMed] [Google Scholar]
- 140.Garnick RL, Le Quesne PW. Biomimetic transformations among monomeric macroline-related indole alkaloids. J Am Chem Soc. 1978;100:4213–4219. [Google Scholar]
- 141.Liao X, Zhou H, Wearing XZ, Ma J, Cook JM. The first regiospecific, enantiospecific total synthesis of 6-oxoalstophylline and an improved total synthesis of alstonerine and alstophylline as well as the bisindole alkaloid macralstonine. Org Lett. 2005;7:3501–3504. doi: 10.1021/ol051208y. [DOI] [PubMed] [Google Scholar]
- 142.Nicolaou K, Baran PS, Zhong YL. Selective oxidation at carbon adjacent to aromatic systems with IBX. J Am Chem Soc. 2001;123:3183–3185. doi: 10.1021/ja004218x. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji J, Nagashima H, Hori K. A new preparative method for 1,3-dicarbonyl compounds by the regioselective oxidation of alpha, beta-unsaturated carbonyl compounds, catalyzed by PdCl2 using hydroperoxides as the reoxidant of Pd0. Chem Lett. 1980:257–260. doi: 10.1246/cl.1980.257. [DOI] [Google Scholar]
- 144.Zhao S, Liao X, Cook JM. Enantiospecific, stereospecific total synthesis of (+)-majvinine, (+)-10-methoxyaffinisine, and (+)-Na-methylsarpagine as well as the total synthesis of the alstonia bisindole macralstonidine. Org Lett. 2002;4:687–690. doi: 10.1021/ol010222h. [DOI] [PubMed] [Google Scholar]
- 145.Zhao S, Liao X, Wang T, Flippen-Anderson J, Cook JM. The enantiospecific, stereospecific total synthesis of the ring-A oxygenated sarpagine indole alkaloids (+)-majvinine, (+)-10-methoxyaffinisine, and (+)-Na-methylsarpagine, as well as the total synthesis of the Alstonia bisindole alkaloid macralstonidine. J Org Chem. 2003;68:6279–6295. doi: 10.1021/jo030055u. [DOI] [PubMed] [Google Scholar]
- 146.Heath-Brown B, Philpott P. Studies in the indole series. Part III. The Japp–Klingemann reaction. J Chem Soc. 1965:7185–7193. [Google Scholar]
- 147.Liao X. PhD Thesis. University of Wisconsin-Milwaukee; Milwaukee, WI, USA: 2007. The First Total Synthesis of the Indole Alkaloids, Macralstonidine, 6-oxoalstophylline, 10-methoxyvellosimine, Lochnerine, Sarpagine and an Improved Total Synthesis of Macralstonine and Macroline, as Well as the Formal Total Synthesis of Dispegatrine. [Google Scholar]
- 148.Hesse M, Hürzeler H, Gemenden C, Joshi B, Taylor W, Schmid H. Die Struktur des Alstonia-Alkaloides Villalstonin Vorläufige Mitteilung. Helv Chim Acta. 1965;48:689–704. doi: 10.1002/hlca.19650480405. [DOI] [PubMed] [Google Scholar]
- 149.Pan L, Terrazas C, Acuña UM, Ninh TN, Chai H, de Blanco EJC, Soejarto DD, Satoskar AR, Kinghorn AD. Bioactive indole alkaloids isolated from Alstonia angustifolia. Phytochem Lett. 2014;10:54–59. doi: 10.1016/j.phytol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nordman C, Kumra S. The structure of villalstonine1. J Am Chem Soc. 1965;87:2059–2060. [Google Scholar]
- 151.Kump W, Schmid H. Über die alkaloide von Pleiocarpa mutica BENTH. Helv Chim Acta. 1961;44:1503–1516. [Google Scholar]
- 152.Kam TS, Subramaniam G, Chen W. Alkaloids from Kopsia dasyrachis. Phytochemistry. 1999;51:159–169. [Google Scholar]
- 153.Bartlett M, Sklar R, Smith A, Taylor W. The alkaloids of Hunteria eburnea Pichon. III. 1 The tertiary bases. J Org Chem. 1963;28:2197–2199. [Google Scholar]
- 154.Hesse M, Philipsborn W, Schumann D, Spiteller G, Spiteller-Friedmann M, Taylor W, Schmid H, Karrer P. Die strukturen von C-fluorocurin, C-mavacurin und pleiocarpamin. 57. Mitteilung über Curare-alkaloide. Helv Chim Acta. 1964;47:878–911. [Google Scholar]
- 155.Mayerl F, Hesse M. Macrocarpamin, ein neues bisindolalkaloid aus Alstonia macrophylla WALL. 167. Mittelung über organische naturstoffe. Helv Chim Acta. 1978;61:337–351. [Google Scholar]
- 156.Said IM, Din LB, Yusoff NI, Wright CW, Cai Y, Phillipson JD. A new alkaloid from the roots of Alstonia angustifolia. J Nat Prod. 1992;55:1323–1324. [Google Scholar]
- 157.Khan ZM, Hesse M, Schmid H. Die Struktur des quartären Alkaloides Macrosalhin. Helv Chim Acta. 1967;50:1002–1010. doi: 10.1002/hlca.19670500403. [DOI] [PubMed] [Google Scholar]
- 158.Gan T, Cook JM. General approach for the synthesis of macroline/sarpagine related indole alkaloids via the asymmetric Pictet-Spengler reaction: The enantiospecific synthesis of (−)-anhydromacrosalhine-methine. Tetrahedron Lett. 1996;37:5033–5036. [Google Scholar]
- 159.Gan T, Cook JM. Partial synthesis of the antiamoebic bisindole alkaloid (−)-macrocarpamine. Tetrahedron Lett. 1996;37:5037–5038. [Google Scholar]
- 160.Gan T, Cook JM. Enantiospecific total synthesis of (−)-anhydromacrosalhine-methine and partial synthesis of the antiamoebic bisindole alkaloid (−)-macrocarpamine. J Org Chem. 1998;63:1478–1483. [Google Scholar]
- 161.Takayama H, Phisalaphong C, Kitajima M, Aimi N, Sakai SI. An efficient synthetic pathway to the macroline-type indole alkaloids, talcarpine and alstonerine from ajmaline. Tetrahedron. 1991;47:1383–1392. [Google Scholar]
- 162.Wearing XZ. PhD Thesis. Univeristy of Wisconsin-Milwaukee; Milwaukee, WI, USA: 2004. Enantiospecific Stereospecific Total Synthesis of the Oxindole Alkaloid (+)-alstonisine and Stereocontrolled Total Synthesis of (−)-11-methoxy-17-epivincamajine as Well as Studies Directed toward the Total Synthesis of Nb-demethylalstophylline Oxindole. [Google Scholar]
- 163.Zhang L, Cook J. General approach to the synthesis of macroline-related alkaloids. Stereospecific total synthesis of (−)-alstonerine. J Am Chem Soc. 1990;112:4088–4090. [Google Scholar]
- 164.Lin M, Yang BQ, Yu DQ. Studies on the quaternary alkaloids of Rauvolfia verticillata (lour.) Baill var. Hainanensis Tsiang. Acta Pharmacol Sin. 1986;21:114–118. [PubMed] [Google Scholar]
- 165.Lin M, Yu DQ, Liu X, Fu F, Zheng Q, He C, Bao G, Xu C. Chemical studies on the quaternary alkaloids of Rauvolfia verticillata (Lour.) Baill. F. ruberocarpa HT Chang. mss. Acta Pharmacol Sin. 1985;20:198–202. [PubMed] [Google Scholar]
- 166.Arbain D, Dachriyanus F, Sargent MV, Skelton BW, White AH. Unusual indole alkaloids from Ophiorrhiza blumeana Korth. J Chem Soc Perkin Trans. 1998;1:2537–2540. [Google Scholar]
- 167.Orazi OO, Corral RA, Stoichevich ME. Studies on plants: XI. Alkaloids of Aspidosperma spegazzinii. Can J Chem. 1966;44:1523–1529. [Google Scholar]
- 168.Madinaveitia A, Valencia E, Bermejo J, Gonzalez A. Indole alkaloids from Rauwolfia sprucei. Biochem Syst Ecol. 1995;23:877. [Google Scholar]
- 169.Edwankar CR, Edwankar RV, Deschamps JR, Cook JM. Nature-inspired stereospecific total synthesis of P-(+)-dispegatrine and four other monomeric sarpagine indole alkaloids. Angew Chem Int Ed. 2012;51:11762–11765. doi: 10.1002/anie.201206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Edwankar CR, Edwankar RV, Namjoshi OA, Liao X, Cook JM. Stereospecific approach to the synthesis of ring-A oxygenated sarpagine indole alkaloids. Total synthesis of the dimeric indole alkaloid P-(+)-dispegatrine and six other monomeric indole alkaloids. J Org Chem. 2013;78:6471–6487. doi: 10.1021/jo400469t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tohma H, Morioka H, Takizawa S, Arisawa M, Kita Y. Efficient oxidative biaryl coupling reaction of phenol ether derivatives using hypervalent iodine (III) reagents. Tetrahedron. 2001;57:345–352. [Google Scholar]
- 172.Larock RC, Yum EK. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J Am Chem Soc. 1991;113:6689–6690. [Google Scholar]
- 173.Larock R, Yum E, Refvik M. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J Org Chem. 1998;63:7652–7662. [Google Scholar]
- 174.Schollkopf U. Asymmetric syntheses of amino acids via metalated bis-lactim ethers of 2,5-diketopiperazines. Pure Appl Chem. 1983;55:1799–1806. [Google Scholar]
- 175.Schöllkopf U, Groth U, Deng C. Enantioselective syntheses of (R)-amino acids using L-valine as chiral agent. Angew Chem Int Ed. 1981;20:798–799. [Google Scholar]
- 176.Ma J, Yin W, Zhou H, Cook JM. Total synthesis of the opioid agonistic indole alkaloid mitragynine and the first total syntheses of 9-methoxygeissoschizol and 9-methoxy-Nb-methylgeissoschizol. Org Lett. 2007;9:3491–3494. doi: 10.1021/ol071220l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kondo Y, Kojima S, Sakamoto T. General and facile synthesis of indoles with oxygen-bearing substituents at the benzene moiety. J Org Chem. 1997;62:6507–6511. [Google Scholar]
- 178.Lizos DE, Murphy JA. Concise synthesis of (±)-horsfiline and (±)-coerulescine by tandem cyclisation of iodoaryl alkenyl azides. Org Biomol Chem. 2003;1:117–122. doi: 10.1039/b208114h. [DOI] [PubMed] [Google Scholar]
- 179.Snieckus V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem Rev. 1990;90:879–933. [Google Scholar]
- 180.Zhou H, Liao X, Cook JM. Regiospecific, enantiospecific total synthesis of the 12-alkoxy-substituted indole alkaloids, (+)-12-methoxy-Na-methylvellosimine, (+)-12-methoxyaffinisine, and (−)-fuchsiaefoline. Org Lett. 2004;6:249–252. doi: 10.1021/ol0362212. [DOI] [PubMed] [Google Scholar]
- 181.Abramovitch R, Shapiro D. 880. Tryptamines, carbolines, and related compounds. Part II. A convenient synthesis of tryptamines and β-carbolines. J Chem Soc. 1956:4589–4592. [Google Scholar]
- 182.Robinson B. The Fischer Indole Synthesis. John Wiley & Sons Ltd; New York, NY, USA: 1982. [Google Scholar]
- 183.Nelson TD, Crouch RD. Cu, Ni, and Pd mediated homocoupling reactions in biaryl syntheses: The Ullmann reaction. Org React. 2004 doi: 10.1002/0471264180.or063.03. [DOI] [Google Scholar]
- 184.Cox ED, Cook JM. The Pictet-Spengler condensation: A new direction for an old reaction. Chem Rev. 1995;95:1797–1842. [Google Scholar]
- 185.Klausen RS, Jacobsen EN. Weak Brønsted acid–thiourea co-catalysis: Enantioselective, catalytic Protio-Pictet–Spengler reactions. Org Lett. 2009;11:887–890. doi: 10.1021/ol802887h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stöckigt J, Antonchick AP, Wu F, Waldmann H. The Pictet–Spengler reaction in nature and in organic chemistry. Angew Chem Int Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- 187.Yang J, Rallapalli SK, Cook JM. The first enantiospecific total synthesis of the 3-oxygenated sarpagine indole alkaloids affinine and 16-epiaffinine, as well as vobasinediol and 16-epivobasinediol. Tetrahedron Lett. 2010;51:815–817. [Google Scholar]
- 188.Solé D, Urbaneja X, Bonjoch J. Palladium-catalyzed intramolecular coupling of amino-tethered vinyl halides with ketones, esters, and nitriles using potassium phenoxide as the base. Adv Synth Catal. 2004;346:1646–1650. [Google Scholar]
- 189.Keller PA, Yepuri NR, Kelso MJ, Mariani M, Skelton BW, White AH. Oxidative coupling of indoles using thallium (III) trifluoroacetate. Tetrahedron. 2008;64:7787–7795. [Google Scholar]
- 190.McKillop A, Turrell AG, Young DW, Taylor EC. Thallium in organic synthesis. 58. Regiospecific intermolecular oxidative dehydrodimerization of aromatic compounds to biaryls using thallium (III) trifluoroacetate. J Am Chem Soc. 1980;102:6504–6512. [Google Scholar]
- 191.Mckillop A, Taylor EC. Recent advances in organothallium chemistry. Adv Organomet Chem. 1973;11:147–206. [Google Scholar]
- 192.Elson IH, Kochi JK. Thallium (III) in one-electron oxidation of arenes by electron spin resonance. J Am Chem Soc. 1973;95:5060–5062. [Google Scholar]
- 193.Lau W, Kochi J. Kinetics and mechanism of aromatic thallation. Identification and proof of competing electrophilic and electron-transfer pathways. J Am Chem Soc. 1984;106:7100–7112. [Google Scholar]