Abstract
Background
Human metapneumovirus (hMPV) causes upper and lower respiratory tract infections (URI and LRI, respectively) in healthy and immunocompromised patients; however, its clinical burden in patients with cancer remains unknown.
Methods
In a retrospective study of all laboratory-confirmed hMPV infections treated at our institution between April 2012 and May 2015, we determined clinical characteristics, risk factors for progression to LRI, treatment, and outcomes in patients with cancer.
Results
We identified 181 hMPV infections in 90 (50%) patients with hematologic malignancies (HM), 57 (31%) hematopoietic cell transplantation (HCT) recipients, and 34 patients (19%) with solid tumors. Most patients (92%) had a community-acquired infection, presented with URI (67%), and 43% developed LRI (59 presented with LRI and 19 progressed from URI to LRI). On multivariable analysis, an underlying HM (adjusted odds ratio [aOR], 3.11(1.12-8.64); P=0.029), nosocomial infection (aOR, 26.9 (2.79-259.75); P=0.004), and hypoxia (SpO2 ≤ 92%) at presentation (aOR, 9.61(1.98-46.57); P = 0.005) were independent factors associated with LRI. All-cause mortality at 30 days from hMPV diagnosis was low (4%) and patients with LRI had a 10% mortality rate at day 30 from diagnosis; whereas, patients with URI had 0% mortality rate.
Conclusions
hMPV infections in patients with cancer may cause significant morbidity, especially for those with underlying HM who may develop an LRI. Despite high morbidity and the lack of directed antiviral therapy for hMPV infections, mortality at day 30 from this infection remained low in this studied population.
Keywords: hMPV, stem cell transplantation, leukemia, cancer, respiratory virus, pneumonia, death
Introduction
In 2001, human metapneumovirus (hMPV), an enveloped nonsegmented negative RNA-Paramyxoviridae virus, was discovered in the Netherlands 1. It has been reported in 4% of adults and 13% of children with community-acquired pneumonia 2-4. The virus can affect all age groups with upper respiratory infections (URI) and lower respiratory tract infections (LRI); however, severe disease has been described in young children 5 and older adults 6. The diagnosis of hMPV from respiratory specimens is dependent upon molecular assays (i.e., reverse transcriptase polymerase chain reaction), which are more sensitive than older methods such as direct fluorescent antibody, viral cultures, and serology.7 In 2012, we adopted a new molecular assay (FilmArray Respiratory Panel, BioFire Diagnostics, LLC) which enhanced the diagnosis of patients with respiratory viral infections secondary to hMPV and other respiratory viruses from respiratory specimens.
hMPV infections in immunocompromised hosts have been described in small case series. In patients with cancer, hMPV incidence is similar to that in the immunocompetent population (approximately 7%) 8,9. hMPV-associated LRI has been reported in as many as 41% of patients with cancer 8 and 100% of children undergoing hematopoietic cell transplantation (HCT) 10. Yet hMPV-associated mortality remains low 8,9,11 unless bronchoalveolar lavage (BAL) findings are positive for the virus 12. These studies were limited by small sample size and inadequate power to determine risk factors and outcomes of hMPV infections in patients with cancer and associated mortality and morbidity.13 Although supportive measures may be in place, hMPV treatment remains a challenge because the only in vitro active drug choice is ribavirin for inhibition of hMPV replication.14,15. In addition, intravenous immunoglobulins (IVIGs) with or without ribavirin have been used in patients with hMPV infections16-18 with lack of systematic evaluation for efficacy. The ECIL 4 European Conference on Infections in Leukemia addressed community-acquired respiratory viruses, including hMPV; however, this therapy lacks systematic evaluation19.
In this large retrospective study, we aimed to determine the clinical characteristics and outcomes of hMPV infections in patients with cancer who are immunocompromised. We attempted to characterize the risk factors associated with development of hMPV-associated LRI, hMPV-associated mortality, and all-cause mortality to identify patients with specific underlying malignancies who are at higher risk for these outcomes and who may be suitable targets for antiviral therapy.
Patients and methods
This study was conducted at the University of Texas MD Anderson Cancer Center in Houston, Texas. The Infection Control database was searched to identify all patients with laboratory-confirmed hMPV infections between April 2012 and May 2015. The BioFire FilmArray Respiratory Panel at our institution was used to diagnose respiratory viral infections including hMPV. The institutional review board approved the protocol, and the waiver for informed consent was granted.
Data collection
We reviewed patient medical records and collected these data: demographics including age, gender, and race; smoking history; cancer type; and cancer status (complete remission or active disease) at the time of infection. For HCT recipients, we reviewed the underlying cancer, the type of transplant (matched-related donor, matched-unrelated donor, haploidentical, mismatched, and autologous), the source (marrow, cord, or peripheral), date of HCT, use of myeloablative versus nonmyeloablative conditioning regimens and type of immunosuppressive therapy used, time of engraftment, history and type of graft-versus-host-disease (GVHD) (acute or chronic), grade and organ involvement, and cytomegalovirus (CMV) serostatus. For hMPV infection episodes, we included coinfections within 30 days prior to and after the hMPV episode, if any; date of symptom onset; type of infection at the time of presentation (community-acquired versus nosocomial acquisition); infection site at presentation (URI versus LRI); absolute neutrophils count (ANC); absolute lymphocytes count (ALC); and gamma globulin levels (when available) up to 30 days before presentation. Systemic steroid use and doses were recorded within 30 days before the infection diagnosis. We also collected data on outcomes and therapy, including whether patients required hospitalization, length of stay if admitted, intensive care unit admission (at onset or later), use of mechanical ventilation, use of ribavirin (oral versus aerosolized form), IVIGs, and date and cause of death. Oxygen saturation at presentation was recorded as well as the lowest oxygen saturation during the infection and the type of oxygen supplementation (nasal cannula, venti-mask, face-mask, vapotherm, or bilevel positive airway pressure).
Definitions
hMPV cases were defined in this study as situations in which a patient with cancer developed acute symptomatic respiratory illness and had a positive nasal wash result and/or BAL finding indicating hMPV. Community-acquired cases occurred when patients developed symptoms while they were outpatients and/or before hospitalization or within the first 5 days after admission 20,21. Symptomatic hMPV infections that develop > 5 days after hospitalization of patients are considered nosocomially-acquired. URI was defined as the development of rhinorrhea, nasal or sinus congestion, otitis media, pharyngitis, cough, or shortness of breath with no hypoxemia or infiltrates on chest radiographic imaging in patients with a positive hMPV test result in a nasal wash. LRI was defined when new or worsening pulmonary infiltrates were seen on chest radiograph and/or when hMPV was detected in a lower respiratory specimen such as endotracheal tube aspirate, sputum, or BAL. Neutropenia was defined as ANC<500/mL and lymphopenia was defined as ALC<200/mL. All-cause mortality was assessed within 30 days and 90 days from hMPV diagnosis and was attributed to hMPV if a patient had persistent or progressive hMPV LRI with respiratory failure at the time of death.
Statistical analysis
We evaluated patient characteristics using descriptive statistics. Categorical variables were compared using the χ2 test or Fisher’s exact test, and continuous variables were compared using the Student t test or Wilcoxon rank sum test. Multivariable logistic regression analyses were used to identify risk factors associated with LRI and reported as adjusted odds ratios (aOR) and 95% confidence intervals (CI). A secondary model restricted to those patients who presented with URI (n=122) was also constructed to identify risk factors for progression from URI to LRI. The probability of progression from URI to LRI between 3 cancer groups (hematologic malignancies [HM], HCT, and solid tumor) was compared using a Kaplan-Meier failure curve. A 2-sided P value of 0.05 was considered statistically significant. All statistical analyses were performed with Stata Software Version 13 (Statacorp, College Station, Texas).
Results
Patients’ characteristics
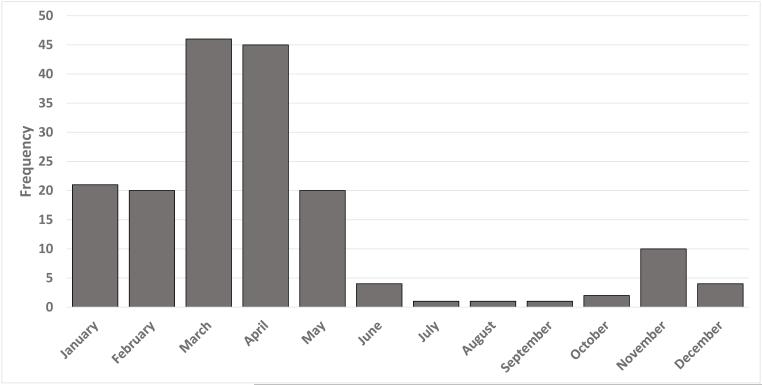
Between April 2012 and May 2015, 181 laboratory-confirmed hMPV infections were identified in patients with cancer; 34 (19%) patients had solid tumors, 57 (31%) patients were HCT recipients (in remission), and 90 (50%) patients had HM. Patients with relapsed HM after HCT were included in the HM group. Patients’ characteristics are depicted in Table 1. The median age was 59 years (range, 1-88 years), and 60% were men. Most patients were non-Hispanic whites (111, 61%) and never smoked (126, 70%). Among patients with HM or post-HCT status, multiple myeloma was the most common underlying malignancy (30%). The majority of patients (92%) had community-acquired infections that were detected throughout the year with a peak during April and May (Figure 1) and had occurred with URI (67%). The overall LRI rate was 43%, and patients with HM had the highest rate of LRI (54%). All-cause mortality at days 30 and 90 from infection diagnosis was 4% and 7%, respectively.
TABLE 1.
Characteristics and Outcomes of 181 Patients With Cancer and Human Metapneumovirus Infections
| No. of Patients (%) | ||||
|---|---|---|---|---|
| Characteristic | Solid Tumors |
HCT, Remission |
HM | Total |
| Total cohort | 34 (19) | 57 (31) | 90 (50) | 181 (100) |
| Age: Median [range] y | 62 [3-86] | 56 [16-76] | 59 [1-88] |
59 [1-88] |
| Sex | ||||
| Male | 16 (47) | 30 (53) | 63 (70) | 109 (60) |
| Female | 18 (53) | 27 (47) | 27 (30) | 72 (40) |
| Racea | ||||
| Non-Hispanic white | 16 (47) | 36 (63) | 59 (66) | 111 (61) |
| Hispanic | 11 (32) | 12 (21) | 16 (18) | 39 (22) |
| Black | 5 (15) | 6 (11) | 8 (9) | 19 (11) |
| Asian | 1 (3) | 2 (3) | 2 (2) | 5 (3) |
| Other | 1 (3) | 1 (2) | 4 (4) | 6 (3) |
| Smokinga | ||||
| Never smoker | 22 (65) | 40 (70) | 64 (71) | 126 (70) |
| Former smoker | 11 (32) | 16 (28) | 22 (24) | 49 (27) |
| Current smoker | 1 (3) | 1 (2) | 3 (3) | 5 (3) |
| Type of malignancy | ||||
| AML | 0 (0) | 12 (21) | 14 (16) | 26 (14) |
| ALL | 0 (0) | 9 (16) | 13 (14) | 22 (12) |
| CML | 0 (0) | 2 (4) | 6 (7) | 8 (4) |
| CLL | 0 (0) | 3 (5) | 4 (4) | 7 (4) |
| Hodgkin lymphoma | 0 (0) | 1 (2) | 5 (6) | 6 (3) |
| NHL | 0 (0) | 11 (19) | 10 (11) | 21 (12) |
| MDS | 0 (0) | 2 (4) | 4 (4) | 6 (3) |
| MM | 0 (0) | 14 (25) | 30 (33) | 44 (24) |
| AA | 0 (0) | 2 (4) | 0 (0) | 2 (1) |
| Other | 34 (100) | 1 (2) | 4 (4 | 39 (22) |
| Type of HCT | ||||
| None | 34 (100) | 0 | 64 (71) | 98 (54) |
| MRD | 0 (0) | 20 (35) | 2 (2) | 22 (12) |
| MUD | 0 (0) | 16 (28) | 3 (33) | 19 (10) |
| Haploidentical | 0 (0) | 3 (5) | 1 (1) | 4 (2) |
| Cord | 0 (0) | 2 (4) | 2 (2) | 4 (2) |
| Mismatched | 0 (0) | 1 (2) | 0 | 1 (1) |
| Autologous | 0 (0) | 15 (26) | 18 (20) | 33 (18) |
| HCT cell source | ||||
| Bone marrow | 0 (0) | 7 (12) | 0 (0) | 7 (4) |
| Cord | 0 (0) | 2 (4) | 2 (2) | 4 (2) |
| Peripheral | 0 (0) | 48 (84) | 24 (27) | 72 (40) |
| Type of infection | ||||
| Community-acquired | 32 (94) | 52 (91) | 82 (91) | 166 (92) |
| Nosocomial | 2 (6) | 5 (9) | 8 (9) | 15 (8) |
| Site of infection at the time of presentation | ||||
| URI | 25 (74) | 43 (75) | 54 (60) | 122 (67) |
| LRI | 9 (26) | 14 (25) | 36 (40) | 59 (33) |
| Progression from URI to LRI | ||||
| No | 24 (71) | 38 (67) | 41 (46) | 103 (57) |
| Yes | 1 (3) | 5 (9) | 13 (14) | 19 (10) |
| Time to progression from URI to LRI: Median [range], db |
1 | 12 [1-30] | 8 [1-30] | 8 [1-30] |
| Overall LRI | ||||
| No | 24 (71) | 38 (67) | 41 (46) | 103 (57) |
| Yes | 10 (29) | 19 (34) | 49 (54) | 78 (43) |
| Steroids within 30 d before hMPV | ||||
| No | 25 (74) | 39 (70) | 52 (58) | 116 (64) |
| Yes | 9 (26) | 18 (32) | 38 (42) | 65 (36) |
| Lymphopeniaa | ||||
| No | 31 (91) | 54 (95) | 75 (83) | 160 (88) |
| Yes | 2 (6) | 3 (5) | 15 (17) | 20 (11) |
| Neutropeniaa | ||||
| No | 29 (85) | 56 (98) | 69 (77) | 154 (85) |
| Yes | 4 (12) | 1 (2) | 21 (23) | 26 (14) |
| Hypoxia at presentation | ||||
| >92% | 29 (85) | 53 (93) | 82 (91) | 164 (91) |
| ≤92% | 5 (15) | 4 (7) | 8 (9) | 17 (9) |
| Ribavirin | ||||
| URI stage | 0 | 1 (2) | 1 (1) | 2 (1) |
| LRI stage | 0 | 2 (4) | 1 (1) | 3 (2) |
| IVIG | ||||
| URI stage | 1 (3) | 7 (13) | 6 (7) | 14 (8) |
| LRI stage | 0 (0) | 3 (5) | 16 (18) | 19 (11) |
| Coinfection before hMPV | ||||
| Pulmonary | 5 (15) | 11 (19) | 15 (7) | 31 (17) |
| Extrapulmonary | 1 (3) | 1 (2) | 6 (7) | 8 (4) |
| Coinfection after hMPV | ||||
| Pulmonary | 3 (9) | 2 (4) | 4 (4) | 9 (5) |
| Extrapulmonary | 0 (0) | 1 (2) | 2 (2) | 3 (2) |
| Hospital admission secondary to infectionc | 15/29 (52) | 21/30 (70) | 44/78 (56) |
80/137 (58) |
| Length of hospital stay: Median [range], dc | 4 [2-20] | 6 [3-17] | 6 [2-29] | 6 [2-29] |
| ICU at onset | 2 (6) | 1 (2) | 1 (1) | 4 (2) |
| ICU later during the illness | 1 (3) | 1 (2) | 4 (4) | 6 (3) |
| Mechanical ventilation | 3 (9) | 2 (4) | 3 (3) | 8 (4) |
| Oxygen supplement | 10 (29) | 12 (21) | 26 (29) | 48 (27) |
| All-cause mortality, 30 d | 1 (3) | 1 (2) | 6 (7) | 8 (4) |
| All-cause mortality, 90 d | 2 (6) | 2 (4) | 8 (9) | 12 (7) |
Abbreviations: AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; HCT, hematopoietic cell transplantation; HM, hematologic malignancy; hMPV, human metapneumovirus; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LRI, lower respiratory tract infection; MDS, myelodysplastic syndrome; MM, multiple myeloma; MRD, matched-related donor; MUD, matched-unrelated donor; NHL, non-Hodgkin lymphoma; URI, upper respiratory tract infection.
One patient was missing information.
This analysis was restricted to patients who progressed from URI to LRI (n = 19).
Analysis of the time to progression excluded patients who were admitted before hMPV diagnosis.
Fig 1. Seasonal distribution of hMPV infections between April 2012 and May 2015 (n = 181).
Abbreviation: hMPV, human metapneumovirus.
hMPV-associated LRI
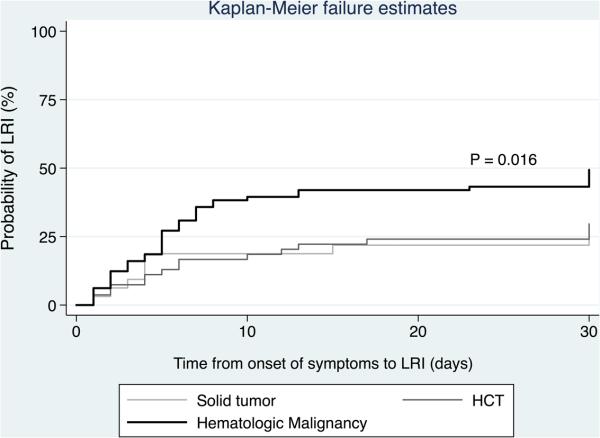
Patient characteristics associated with LRI are depicted in Table 2. Patients with LRI were more likely than patients with URI to have HM (adjusted odds ratio [aOR], 3.11; 95% confidence interval [CI], 1.12-8.64; P = 0.029), nosocomially-acquired hMPV(aOR, 26.9; 95% CI, 2.79-259.75; P = 0.004), and hypoxia (SpO2 ≤ 92%) at presentation (aOR, 9.61; 95% CI, 1.98-46.57; P = 0.005). When the logistic model was restricted to only patients who presented with URI, having an underlying HM was a significant predictor for progression to LRI (aOR, 27.23; 95% CI, 1.44-514.82; P = 0.028), as did having nosocomially-acquired infections(aOR, 500.41; 95% CI, 15.79-15854.59; P < 0.001). The Kaplan-Meier failure curve showed a significantly higher incidence of LRI in the HM group versus the solid tumor or HCT groups (P = 0.016) (Figure 2). Age, gender, smoking status, immunodeficiency status based on ANC and ALC values, steroid use, and presence of pulmonary copathogens prior to hMPV diagnosis did not significantly affect progression to LRI in this cohort.
TABLE 2.
Patient Characteristics Associated With Human Metapneumovirus Lower Respiratory Tract Infection
| No. (%) | Total Cohort, n = 178a | Restricted to Patients who Presented With URI, n = 122 |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | URI | LRI | Unadjuste d OR [95% CI] |
P | Adjusted OR [95% CI] |
P | Adjusted OR [95% CI] |
P |
| All patients | 103 (57) |
78 (43) |
||||||
| Age: Median/range, yb |
59/1- 84 |
59/7- 88 |
1.08/0.93- 1.26 |
.28 8 |
1.15/0.94-1.39 | .16 7 |
1.06/0.77-1.4 5 |
.71 8 |
| Sex | ||||||||
| Men | 59 (54) |
50 (46) |
1.00 | |||||
| Women | 44 (61) |
28 (39) |
0.75 [0.41-1.38 ] |
.35 4 |
||||
| Race | ||||||||
| Non-Hispanic white |
55 (50) |
56 (50) |
1.00 | 1.00 | ||||
| Hispanic | 27 (69) |
12 (31) |
0.44 [0.2-0.95] |
.03 6 |
0.46 [0.18-1.13] | .09 1 |
0.54 [0.08-3.51] |
.52 1 |
| Black | 12 (63) |
7 (37) |
0.57 [0.21-1.56 ] |
.27 7 |
0.64 [0.2-2.10] | .46 4 |
4.6 [0.84-25.38] |
.07 9 |
| Asian/other | 9 (82) |
2 (18) |
0.21 [0.05-1.06 ] |
.05 8 |
0.15 [0.03-0.87] | .03 5 |
0.56 [0.05-6.3] |
.63 5 |
| Smoking | ||||||||
| Never | 71 (56) |
55 (44) |
1.00 | 1.00 | ||||
| Former/current smoker |
31 (57) |
23 (43) |
0.96 [0.5-1.82] |
.89 6 |
0.75 [0.34-1.65] | .47 5 |
1.19 [0.3-4.77] |
.80 4 |
| Underlying condition |
||||||||
| Solid tumor<zaq;4> |
24 (71) |
10 (29) |
1.00 | 1.00 | ||||
| HCT, in remission |
38 (67) |
19 (33) |
1.2 [0.48-3.01 ] |
.69 8 |
1.09 [0.37-3.23] | .87 7 |
4.35 [0.23-83.51] |
.33 |
| HM | 41 (46) |
49 (54) |
2.89 [1.23-6.69 ] |
.01 5 |
3.11 [1.12-8.64] | .02 9 |
27.23 [1.44-514.82 ] |
.02 8 |
| Type of infection | ||||||||
| Community-ac quired |
102 (61) |
64 (39) |
1.00 | 1.00 | 1.00 | |||
| Nosocomial | 1 (7) | 14 (93) |
22.31 [2.86-173. 79] |
.00 3 |
26.9 [2.79-259.75]<z aq;5> |
.00 4 |
500.41 [15.79-15,85 4.59] |
< .00 1 |
| Steroids | ||||||||
| No | 70 (60) |
46 (40) |
1.00 | 1.00 | ||||
| Yes | 33 (51) |
32 (49) |
1.48 [0.8-2.72] |
.21 3 |
0.89 [0.42-1.90] | .76 9 |
0.35 [0.08-1.64] |
.18 4 |
| Immunodeficien cy |
||||||||
| None | 86 (61) |
56 (39) |
1.00 | 1.00 | 1.00 | |||
| Neutropenia | 9 (50) |
8 (50) |
1.54 [0.57-4.11 ] |
.39 3 |
0.47 [00.12-1.79] |
.26 8 |
0.16 [0-5.11] | .29 9 |
| Lymphopenia | 5 (42) |
7 (58) |
2.15 [0.65-7.11 ] |
.21 0 |
1.16 [0.24-5.62] | .85 3 |
1.59 [0.13-19.44] |
.71 7 |
| Both | 2 (25) |
6 (75) |
4.61 [0.89-23.6 4] |
.06 7 |
3.39 [0.44-26.43] |
.24 4 |
3.21 [0.13-79.16] |
.47 6 |
| Hypoxia at presentation |
||||||||
| >92% | 89 (58) |
64 (42) |
1.00 | 1.00 | ||||
| ≤92% | 3 (25) |
9 (75) |
4.17 [1.09-16.0 2] |
.03 7 |
9.61 [1.98-46.57] |
.00 5 |
10.08 [0.55-184.45 ] |
.11 9 |
| IVIG at URI stage |
||||||||
| No | 92 (55) |
75 (45) |
1.00 | — | — | 1.00 | ||
| Yes | 11 (79) |
3 (21) |
0.33 [0.09-1.24 ] |
.10 2 |
— | — | 0.6 [0.08-4.61] |
.62 3 |
| Pulmonary copathogen before hMPV diagnosis |
||||||||
| None | 90 (60) |
60 (40) |
1.00 | 1.00 | ||||
| Pulmonary | 13 (42) |
18 (58) |
2.08 [0.95-4.55 ] |
.06 8 |
1.69 [0.64-4.44] | .28 9 |
0.22 [0.02-2.57] |
.22 6 |
Abbreviations: CI, confidence internal; HCT, hematopoietic cell transplant; HM, hematologic malignancy; hMPV, human metapneumovirus; IVIG, intravenous immunoglobulin; LRI, lower respiratory tract infection; OR, odds ratio; URI, upper respiratory tract infections.
Complete information on all variables included in the model was available for 178 patients.
Age was categorized into 10-year intervals.
Fig 2. Kaplan-Meier failure curve for progression to LRI over time (restricted to patients presenting with URI).
Abbreviations: HCT, hematopoietic cell transplantation; LRI, lower respiratory tract infection.
Among the 78 patients with LRI, BAL was performed on 33 (42%) and hMPV was detected in 23 (70%). Escherichia coli (E. coli) was detected in 1 patient, and no pathogens were detected in the remaining 9 patients. Copathogens were recovered from BAL samples of only 7 patients with LRI and hMPV positivity and included Cytomegalovirus (1), E. coli (1), methicillin-resistant Staphylococcus aureus (S. aureus) (1), methicillin-sensitive S. aureus (1), parainfluenza virus (PIV) 3 (1), respiratory syncytial virus (RSV) (1), coronavirus 229E (1), and Arthrographis (1).
All-cause and hMPV-associated mortality
Twelve patients died at a median of 15 days (range, 1-45 days) within their hMPV diagnosis, and mortality rates were similar for all 3 cancer groups. Of these, 4 patients had probable hMPV attributed death (3 with relapsed or refractory HM and 1 matched unrelated donor [MUD] HCT recipient) after progression to respiratory failure within 18 days of hMPV diagnosis (range, 5-36 days). Only 2 patients with hMPV-associated death had pulmonary coinfections, 1 with Stenotrophomonas maltophilia and the other with Aspergillus terreus. The remaining 8 patients died from cancer relapse (4) and other causes (4) at a median of 22 days (range, 1-45 days).
Antiviral therapy (ribavirin and IVIG)
Five patients received ribavirin therapy (2 at the URI stage and 3 at the LRI stage), and 31 patients received IVIG (14 at the URI stage and 17 at the LRI stage). Of the 4 patients who died with respiratory failure, 1 received IVIG and 1 received aerosolized ribavirin with IVIG at the LRI stage, while the others had not received antiviral therapy.
Airflow decline
Pulmonary function tests after infection were performed in 22 HCT recipients (16 allogeneic HCT and 6 autologous HCT) at an average of 60 days (range, 18-520 days) from hMPV diagnosis. Evidence of airflow decline, defined as a drop of at least 15% in forced expiratory volume (FEV1) from pre-HCT to post-infection, was observed in 8 (36%) patients (4 who had matched related donor HCT, 3 who had matched unrelated donor, and 1 who had autologous HCT). The median delta drop in FEV1 was 26% (range, 16%-49%) within a median of 56 days (range, 33-307 days) after hMPV infection. Five of these patients had LRI; however, all patients survived.
Gamma globulin levels
In a subgroup of 39 HCT recipients who had gamma globulin levels checked at the time of presentation, significantly higher levels of gamma globulin levels were observed in patients with URI (1032 mg/dL ± 561) versus patients with LRI (566 mg/dL ± 197; P = 0.01).
Discussion
In this retrospective study of hMPV infections in 181 patients with cancer, we report a high incidence of LRI (43%) and low overall mortality (7%) following these infections. Risk factors associated with LRI were underlying HM, nosocomially-acquired infection, and hypoxia at presentation. Patients with HM were more likely to progress from URI to LRI than HCT recipients or patients with solid tumors. All patients who died within 30 days from hMPV diagnosis had developed LRI (a 10% mortality rate in patients with LRI versus 0% in patients with URI).
An overall LRI rate of 43% was observed in these patients with cancer. This finding is higher when measured against previous studies, which reported an LRI rate of 28% to 41% in patients with cancer and an hMPV diagnosis 22. Incidence of hMPV LRI was consistent with that reported for other respiratory viruses among HCT recipients and patients with HM 22,23. Several risk factors associated with LRI were identified. Hypoxia at the time of diagnosis was a substantial risk factor in the multivariable analysis. Hypoxia and a supplemental oxygen requirement at diagnosis are associated with a higher rate of LRI and death in patients with cancer who have RSV, PIV, and influenza 23. Oxygen use may be a surrogate marker of the extent of lung injury, which may lead to poor outcomes 24. Of interest, an underlying diagnosis of HM significantly increased risk for LRI. Currently, there are limited data on hMPV infections in patients with HM and their impact; however, underlying HM was reported as a significant risk factor for progression to LRI in PIV-associated respiratory infections 25. Patients with HM might have a high level of immunosuppression owing to active chemotherapy at the time of hMPV infection or due to their underlying relapse or refractory disease with subsequent prolonged cytopenias when compared to engrafted HCT recipients in remission.26,27 Nosocomial acquisition of hMPV was associated with significantly higher risk for hMPV-associated LRI. A substantial number of nosocomial hMPV infections also was described in a previous hMPV study of patients with HM 8. Respiratory viruses can be transmitted from either asymptomatic or symptomatic patients, family members, or healthcare workers. This highlights the importance of infection control measures because nosocomial infections were associated with higher morbidity rates in this study’s population.
Neutropenia and/or lymphopenia have been described as major risk factors for LRI and death associated with other respiratory viruses 22,23. This was not observed in our study or other studies examining patients with cancer who had hMPV and can be explained by the fact that only a few patients had these risk factors 8. Similarly, other risk factors for progression to LRI and death (i.e., older age, smoking history, and steroid use) reported with other respiratory viruses were not observed in this population with hMPV infection.
An overall mortality rate of 4% at day 30 and 7% at day 90 is consistent with previous smaller case series in patients with cancer 8,9,11,28,29. Also, hMPV-associated death was only 2%. When evaluated against other respiratory viral infections in patients with cancer (i.e., RSV or PIV), the lower incidence of mortality associated with hMPV infections in our patients with cancer suggests a difference in viral factors (genotype, viral fitness, or virulence) rather than host factors, and further study is warranted.
Ribavirin, which is mainly used to treat RSV infections in HCT recipients 23,30-32, has shown in vitro activity against hMPV by a direct antiviral effect 14 and in mouse models by reducing viral replication 15. In a few case reports, the use of ribavirin was associated with good outcomes following severe hMPV infections in patients with cancer 17,33-35. In our study, the mortality rate remained low despite the lack of ribavirin use in most of the patients and those with LRI in particular.
In a subgroup analysis of 39 HCT recipients who had gamma globulin levels checked at the time of presentation, we observed significantly higher levels of gamma globulins in patients with URI than in those with LRI. Gamma globulin levels are much lower in patients with chronic lymphocytic leukemia (CLL) who have a history of any infection than in patients with CLL with no history of infection 36. This suggests that higher gamma globulin levels can protect against worse outcomes. Standard IVIG administration can inhibit hMPV replication in vitro 14, so we hypothesize that IVIG administration may be beneficial in HCT recipients to prevent progression from hMPV URI to LRI; however, an association between IVIG administration and LRI prevention could not be shown in our study and needs to be systematically determined in future trials. We did not identify other significant risk factors such as age, smoking status, levels of immunodeficiency (ANC, ALC), type of conditioning regimen, and CMV serostatus of the donor or recipient, GVHD, time of engraftment, HCT cell source, or HCT recipient exposure to steroids. In a 2015 study, use of at least 1mg/kg of steroids within 2 weeks leading to diagnosis was the only significant risk factor identified for progression to LRI according to a multivariate regression analyses of 118 HCT recipients 37.
This retrospective study has many limitations including the lack of hMPV quantification in respiratory secretions, which can indicate disease severity as seen in hMPV studies in populations for which higher viral loads have been associated with increased risk for LRI and hospitalization 38,39. Several studies suggest that severity of disease and symptom manifestations varies with hMPV genotype 40,41, but this information was not available in our cohort.
For patients with cancer, the burden of hMPV infection is similar to the burden associated with other respiratory viral infections. However, the mortality rate following hMPV infections is lower than mortality associated with other related viruses such as RSV. Patients with HM, nosocomial infections, and hypoxia at presentation should be closely monitored for risk of progression to LRI. Because hMPV may be acquired nosocomially, leading to worse outcomes and high morbidity, strict adherence to infection control measures and universal hand hygiene should be underscored. The significance of gamma globulin levels and the role of IVIG in prevention of hMPV-associated LRI and/or mortality should be determined in future studies, especially among HCT recipients and patients with HM.
Acknowledgements
We thank Ms Brenda Moss-Feinberg, Department of Scientific Publications at the University of Texas MD Anderson Cancer Center, for her editorial support.
The study was supported in part by the NIH/NCI under award number P30CA016672 and used the Cancer Center Support Grant resources.
Footnotes
Financial Disclosures: All authors declare no competing financial interests.
Conflicts of Interest: The authors declare no conflict of interest.
Author Contributions: F.E.C., D.P.S., and R.F.C. conceptualized and designed the study. F.E.C. and J.K. performed clinical research and data collection. R.F.C., E.A.H., V.M. and C.H. helped with data acquisition. D.P.S. performed the statistical analyses. F.E.C., D.P.S., and R.F.C. wrote the paper. All authors helped critically review the manuscript and checked the final version of it. F.E.C., D.P.S. and R.F.C. are responsible for overall content of the manuscript as guarantors.
The authors have no financial conflicts of interests to declare.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S, Self WH, Wunderink RG, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin Infect Dis. 2008;46(4):571–574. doi: 10.1086/526776. [DOI] [PubMed] [Google Scholar]
- 5.Williams JV, Tollefson SJ, Heymann PW, Carper HT, Patrie J, Crowe JE. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115(6):1311–1312. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44(9):1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 7.Poritz MA, Blaschke AJ, Byington CL, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6(10):e26047. doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192(6):1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamboj M, Gerbin M, Huang CK, et al. Clinical characterization of human metapneumovirus infection among patients with cancer. J Infect. 2008;57(6):464–471. doi: 10.1016/j.jinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan A, Gu Z, Smith T, et al. Prospective detection of respiratory pathogens in symptomatic children with cancer. Pediatr Infect Dis J. 2013;32(3):e99–e104. doi: 10.1097/INF.0b013e31827bd619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11(10):781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144(5):344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 13.Shah DP, Shah PK, Azzi JM, El Chaer F, Chemaly RF. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer Letters. 2016;379(1):100–106. doi: 10.1016/j.canlet.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60(1):51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 15.Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50(2):774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SY, Baek S, Lee SO, et al. Efficacy of oral ribavirin in hematologic disease patients with paramyxovirus infection: analytic strategy using propensity scores. Antimicrob Agents Chemother. 2013;57(2):983–989. doi: 10.1128/AAC.01961-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shachor-Meyouhas Y, Ben-Barak A, Kassis I. Treatment with oral ribavirin and IVIG of severe human metapneumovirus pneumonia (HMPV) in immune compromised child. Pediatr Blood Cancer. 2011;57(2):350–351. doi: 10.1002/pbc.23019. [DOI] [PubMed] [Google Scholar]
- 18.Egli A, Bucher C, Dumoulin A, et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40(6):677–684. doi: 10.1007/s15010-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC Outbreaks of human metapneumovirus in two skilled nursing facilities -West Virginia and Idaho, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62(46):909–913. [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9(6):628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis. 2011;24(4):333–343. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl 5):S344–351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waghmare A, Campbell AP, Xie H, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57(12):1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119(12):2738–2745. doi: 10.1182/blood-2011-08-371112. quiz 2969. [DOI] [PubMed] [Google Scholar]
- 26.Perkins JG, Flynn JM, Howard RS, Byrd JC. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer. 2002;94(7):2033–2039. [PubMed] [Google Scholar]
- 27.Molteni A, Nosari A, Montillo M, Cafro A, Klersy C, Morra E. Multiple lines of chemotherapy are the main risk factor for severe infections in patients with chronic lymphocytic leukemia with febrile episodes. Haematologica. 2005;90(8):1145–1147. [PubMed] [Google Scholar]
- 28.Oliveira R, Machado A, Tateno A, Boas LV, Pannuti C, Machado C. Frequency of human metapneumovirus infection in hematopoietic SCT recipients during 3 consecutive years. Bone Marrow Transplant. 2008;42(4):265–269. doi: 10.1038/bmt.2008.153. [DOI] [PubMed] [Google Scholar]
- 29.Ali M, Baker JM, Richardson SE, Weitzman S, Allen U, Abla O. Human metapneumovirus (hMPV) infection in children with cancer. J Pediatr Hematol Oncol. 2013;35(6):444–446. doi: 10.1097/MPH.0b013e31828ac89c. [DOI] [PubMed] [Google Scholar]
- 30.Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah DP, Ghantoji SS, Mulanovich VE, Ariza-Heredia EJ, Chemaly RF. Management of respiratory viral infections in hematopoietic cell transplant recipients. Am J Blood Res. 2012;2(4):203–218. [PMC free article] [PubMed] [Google Scholar]
- 32.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 33.Bonney D, Razali H, Turner A, Will A. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br J Haematol. 2009;145(5):667–669. doi: 10.1111/j.1365-2141.2009.07654.x. [DOI] [PubMed] [Google Scholar]
- 34.Hofmeyr A, Dunlop L, Ling S, Fiakos E, Maley M. Ribavirin treatment for human metapneumovirus and methicillin-resistant Staphylococcus aureus co-infection in adult haematological malignancy. Clinical Microbiology and Infection. 2011;17:S812. [Google Scholar]
- 35.Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13(3):324–328. doi: 10.1111/j.1399-3062.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visentin A, Compagno N, Cinetto F, et al. Clinical profile associated with infections in patients with chronic lymphocytic leukemia. Protective role of immunoglobulin replacement therapy. Haematologica. 2015;100(12):e515–518. doi: 10.3324/haematol.2015.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo Sachiko, Ted Cooley JMK, Stednick Zach, Jerome Keith R., Englund Janet A., Boeckh Michael J. Human Metapneumovirus Infections in Hematopoietic Cell Transplant Recipients: Seasonality and Factors Associated with Progression to Lower Respiratory Tract Disease. In: Fred Hutchinson Cancer Research Center S, WA, editor. Biology of Blood and Marrow Transplantation. 2015. [Google Scholar]
- 38.Bosis S, Esposito S, Osterhaus AD, et al. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42(3):286–290. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62(4):382–388. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papenburg J, Hamelin M, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206(2):178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Differences in clinical severity between genotype A and genotype B human metapneumovirus infection in children. Clin Infect Dis. 2006;42(12):e111–113. doi: 10.1086/504378. [DOI] [PubMed] [Google Scholar]