Figure 3.
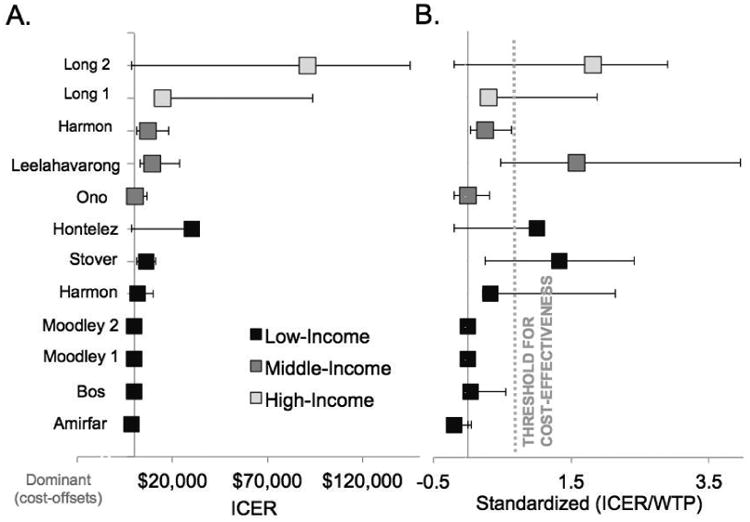
Cost-effectiveness studies of HIV vaccines. A) Incremental cost-effectiveness ratio results from the base case of each study reviewed with error bars representing lower and upper ranges from the sensitivity analysis; B) same as Panel A with ICER standardized to willingness-to-pay threshold specified by study (see Table 1).
Notes: The ICER uncertainty from Hontelez et al. is reported in one direction as a result of the threshold analysis method to set the vaccine price, resulting in an ICER equal to the country-specific willingness-to-pay. Three authors are included twice to reflect different results from multiple publications (Long and Moodley) while another presented results for two populations within one publication (Harmon). Amirfar and Stover did not explicitly state cost-effectiveness thresolds. The threshold from Harmon et al. was applied to the Stover study as both model the same 26 countries. Standardized ICER = ICER/WTP. ICER, Incremental Cost-Effectiveness Ratio. WTP, willingness to pay per health unit gained.
